Characterization of C3’ Epimerization of the Thioheptose Core in the Biosynthesis of Albomycin δ2 Catalyzed by the Radical S-Adenosylmethionine Enzyme AbmJ
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
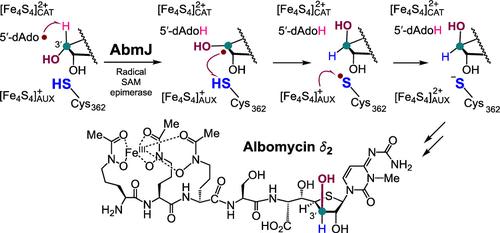
Albomycin δ2 isolated from species of Streptomyces is an antibiotic notable for its oligopeptidyl thionucleoside structure. Its biosynthesis has garnered significant interest, particularly regarding the formation of its unique d-xylo-thioheptose core. The radical S-adenosylmethionine enzyme AbmJ has been shown to catalyze C3′-epimerization of the d-ribo-thiofuranose ring, thereby generating the d-xylo-thioheptose core. However, the catalytic mechanism underlying this biotransformation remains uncharacterized. Hence, a systematic approach was taken to gain insight into AbmJ catalysis. The findings presented herein rule out a dehydrogenation route for epimerization and provide firm evidence supporting a unified mechanism for this class of enzymes. Namely, the reactive 5′-deoxyadenosyl radical, which is derived from the reductive cleavage of SAM, is shown to abstract the C3′ hydrogen from the pro-SB-217452 substrate. The nascent substrate radical is subsequently quenched by a hydrogen-atom transfer to C3′ from the opposite face, thereby completing epimerization. The highlights of this study include the identification of Cys362 as the hydrogen donor in the AbmJ reaction and the discovery that AbmJ can also catalyze epimerization when the C3′-hydroxyl group is replaced with fluorine. These results not only unravel a direct radical quenching mechanism for AbmJ-catalyzed epimerization but also demonstrate that radical SAM-catalyzed stereoinversion is not limited to carbinols.
自由基s -腺苷蛋氨酸酶AbmJ催化合成Albomycin δ2过程中硫庚糖核C3′外映化的表征
从链霉菌中分离出的Albomycin δ2是一种以其寡肽硫代核苷结构而闻名的抗生素。它的生物合成引起了极大的兴趣,特别是关于其独特的d-木基硫庚糖核心的形成。自由基s -腺苷甲硫氨酸酶AbmJ被证明可以催化d-核糖-硫代铀糖环的C3 ' -外映,从而生成d-木基-硫代庚糖核心。然而,这种生物转化的催化机制仍然不清楚。因此,采用系统的方法来深入了解AbmJ的催化作用。本文提出的研究结果排除了外聚化的脱氢途径,并为这类酶的统一机制提供了有力的证据。也就是说,活性的5 ‘ -脱氧腺苷自由基,来源于SAM的还原裂解,被证明从pro-SB-217452底物中提取C3 ’氢。新生的底物自由基随后被氢原子从相反面转移到C3 '而淬灭,从而完成外显异构化。本研究的亮点包括确定了Cys362是AbmJ反应中的氢供体,并发现当C3 ' -羟基被氟取代时,AbmJ也可以催化外映异构化。这些结果不仅揭示了abmj催化外聚反应的直接自由基猝灭机制,而且表明自由基sam催化的立体转化并不局限于甲醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


