Effect of Iron Particle Formation on the Transformation of Al in Drinking Water Distribution System: Implications for Al Deposition Control
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Aluminum (Al), a common residual in drinking water distribution systems (DWDS), can undergo transformation and accumulation, posing challenges for long-term water quality management. However, the interactions between various Al species and iron oxides (FeOx) generated from pipe corrosion remain insufficiently characterized. This study quantified the transformation efficiency of Al under different FeOx formation pathways and water chemistry conditions. In-situ formed FeOx significantly enhanced the transformation of inorganic Al into stable particulate forms, with up to 81.8% reduction in soluble Al after 48 hours, compared to only 18.0% with preformed FeOx at an initial Al concentration of 200 μg/L. Inorganic Al was substantially incorporated into FeOx particles during in-situ formation through isomorphic substitution into layered double hydroxide (LDH) structures, resulting in stable, particle-associated Al. In contrast, preformed FeOx exhibited limited Al adsorption capacity, forming less stable surface associations. Notably, organically complexed Al exhibited strong resistance to transformation and largely remained in the dissolved phase, while also inhibiting FeOx formation. Acidic conditions (pH 4.5) triggered significant Al release from preformed FeOx-Al composites. Elevated pH and increased dissolved organic carbon (DOC) concentrations further promoted Al remobilization, particularly from surfaces of preformed FeOx. Validation experiments using actual tap water confirmed the dominant role of in-situ FeOx formation in promoting the immobilization of inorganic Al into particulate form. These findings provide critical mechanistic insights into FeOx-mediated Al transformation pathways and inform strategies for mitigating Al accumulation and remobilization in DWDS.
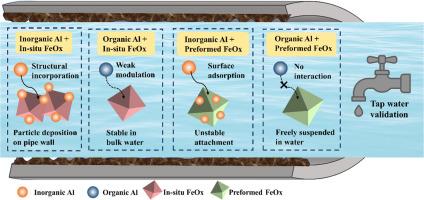
铁颗粒形成对饮用水配水系统中铝转化的影响:对铝沉积控制的启示
铝(Al)是饮用水配水系统(DWDS)中常见的残留物质,它会发生转化和积累,给长期水质管理带来挑战。然而,各种Al与管道腐蚀产生的氧化铁(FeOx)之间的相互作用仍然没有得到充分的表征。本研究量化了不同FeOx形成途径和水化学条件下Al的转化效率。原位形成的FeOx显著促进了无机Al向稳定颗粒形式的转变,在初始Al浓度为200 μg/L时,48小时后可溶性Al的还原率高达81.8%,而预先形成的FeOx仅为18.0%。在原位形成过程中,无机铝通过同构取代成层状双氢氧化物(LDH)结构,大量结合到FeOx颗粒中,形成稳定的颗粒结合的Al。相比之下,预制的FeOx表现出有限的Al吸附能力,形成不稳定的表面结合。值得注意的是,有机络合Al表现出很强的转化抗性,大部分保持在溶解相,同时也抑制了FeOx的形成。酸性条件(pH值为4.5)会导致预制FeOx-Al复合材料释放出大量Al。pH值的升高和溶解有机碳(DOC)浓度的增加进一步促进了Al的再活化,尤其是预成型FeOx表面的再活化。使用自来水的验证实验证实了原位形成FeOx在促进无机Al固定成颗粒形式方面的主导作用。这些发现为feox介导的Al转化途径提供了关键的机制见解,并为减轻DWDS中Al的积累和再动员提供了策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


