Linker modifications in radiolabeled RM26-based antagonists to gastrin-releasing peptide receptor (GRPR) improved tracers’ pharmacokinetics
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
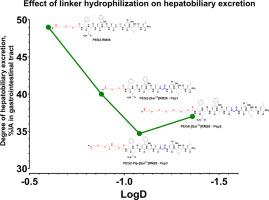
Radionuclide targeting of gastrin-releasing peptide receptor (GRPR) holds significant diagnostic and therapeutic potential, particularly in PSMA-negative/low-grade prostate cancer, estrogen receptor-positive breast cancer, and other malignancies. Recently, our group reported the development and results of the Phase I clinical evaluation of [99mTc]Tc-maSSS-PEG2-RM26, an antagonist GRPR-targeting SPECT imaging agent. This study focuses on developing the next generation of RM-26-based GRPR antagonists with enhanced metabolic stability and improved pharmacokinetics. Four new RM-26-based agents containing sarcosine (Sar) at position 11 to improve the in vivo stability and with more hydrophilic linkers were designed: Pep1 – maSSS-PEG2-[Sar11]RM26, Pep2 – maSSS-PEG6-[Sar11]RM26, Pep3 – maSSS-PEG2-Pip-[Sar11]RM26, and Pep4 – maSSS-EEE-[Sar11]RM26. These analogs were compared both in vitro and in vivo with [99mTc]Tc-maSSS-PEG2-RM26 as a reference. In PC-3 cells, [99mTc]Tc-Pep1, [99mTc]Tc-Pep2 and [99mTc]Tc-Pep3, but not [99mTc]Tc-Pep4, specifically bound to GRPR and exhibited low nanomolar affinity. When compared in vivo, [99mTc]Tc-Pep1, [99mTc]Tc-Pep2, and [99mTc]Tc-Pep3 demonstrated rapid blood clearance with different degrees of hepatobiliary excretion, particularly [99mTc]Tc-Pep2 and [99mTc]Tc-Pep3 had significantly lower activity uptake in the liver and gastrointestinal tract compared to [99mTc]Tc-maSSS-PEG2-RM26. Both [99mTc]Tc-Pep2 and [99mTc]Tc-Pep3 showed improved metabolic stability, bound specifically to GRPR in vivo, and demonstrated a tendency (not statistically significant) for higher tumor activity uptake compared to the reference peptide. Biodistribution data were confirmed by SPECT/CT imaging. In conclusion, the analogs with an elongation of the PEG2-linker either up to PEG6 (Pep2) or with the addition of a basic piperidine-containing moiety (Pep3) demonstrated an improvement of the pharmacokinetic properties of these agents and justify further investigations.
放射性标记的rm26型胃泌素释放肽受体(grpr)拮抗剂的连接子修饰改善了示踪剂的药代动力学。
放射性核素靶向胃泌素释放肽受体(GRPR)具有重要的诊断和治疗潜力,特别是在psma阴性/低级别前列腺癌、雌激素受体阳性乳腺癌和其他恶性肿瘤中。最近,我们小组报道了[99mTc] tc - mass - peg2 - rm26(一种靶向grpr的拮抗剂)的开发和I期临床评估结果。本研究的重点是开发下一代基于rm -26的GRPR拮抗剂,增强代谢稳定性和改善药代动力学。为提高体内稳定性,设计了4种含有11位sarcos (Sar)的rm -26型新制剂:Pep1 - mass - peg2 -[Sar11]RM26、Pep2 - mass - peg6 -[Sar11]RM26、Pep3 - mass - peg2 - pip -[Sar11]RM26和Pep4 - mass - eee -[Sar11]RM26。以[99mTc] tc - mass - peg2 - rm26为参比,对这些类似物进行体外和体内比较。在PC-3细胞中,[99mTc]Tc-Pep1、[99mTc]Tc-Pep2和[99mTc]Tc-Pep3特异性结合GRPR,表现出低纳摩尔亲和力,但[99mTc]Tc-Pep4没有特异性结合。在体内比较,[99mTc]Tc-Pep1、[99mTc]Tc-Pep2和[99mTc]Tc-Pep3表现出快速的血液清除和不同程度的肝胆排泄,特别是[99mTc]Tc-Pep2和[99mTc]Tc-Pep3在肝脏和胃肠道中的活性摄取明显低于[99mTc] tc - mass - peg2 - rm26。[99mTc]Tc-Pep2和[99mTc]Tc-Pep3均表现出更好的代谢稳定性,在体内与GRPR特异性结合,并且与参考肽相比,显示出更高的肿瘤活性摄取趋势(无统计学意义)。生物分布数据通过SPECT/CT成像证实。综上所述,peg2连接体延伸至PEG6 (Pep2)或添加碱性含哌啶片段(Pep3)的类似物表明,这些药物的药代动力学特性得到改善,值得进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


