Molecularly Engineered Ni–O–C Interfacial Sites on g-C3N4 for Efficient Electrocatalytic Urea Synthesis from CO2 and Nitrate
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
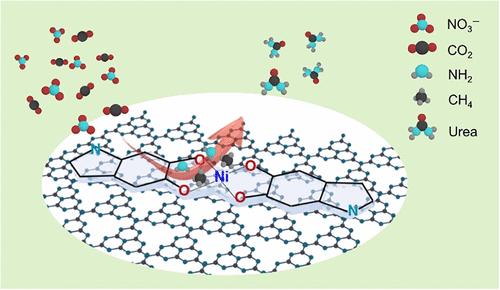
Nickel-based catalysts hold promise for urea electrosynthesis but often suffer from strong chemisorption of CO2, poor stability, and uncontrolled structural evolution under reaction conditions. In this study, we report a molecularly engineered electrocatalyst, Ni-PDA@g-C3N4, that contains highly dispersed Ni–O–C interfacial sites for the efficient and selective synthesis of urea from CO2 and nitrate under ambient conditions. The catalyst is synthesized by chelating Ni2+ ions with polydopamine (PDA) and anchoring them onto graphitic carbon nitride (g-C3N4), forming a robust hybrid interface. This architecture promotes the concurrent activation of CO2 and NO3–, thereby facilitating C–N bond formation. The Ni-PDA@g-C3N4 catalyst achieved a urea yield of 1190.3 μg h–1 mgcat–1 with a Faradaic efficiency of 18.03% and a nitrogen selectivity of 55.78% at −1.3 V vs RHE, along with excellent long-term stability. Density functional theory (DFT) calculations demonstrate that the Ni–O–C interface substantially reduces the energy barriers for key intermediates such as *NHO and *COOH, thus accelerating the C–N coupling process. This work highlights a molecular and interfacial design strategy that offers a promising route toward sustainable urea production from CO2 and nitrogenous wastes.
g-C3N4上分子工程Ni-O-C界面位点用于CO2和硝酸盐高效电催化尿素合成
镍基催化剂在尿素电合成中具有广阔的应用前景,但在反应条件下往往存在CO2化学吸附强、稳定性差、结构演化不受控制等问题。在这项研究中,我们报道了一种分子工程电催化剂Ni-PDA@g-C3N4,它含有高度分散的Ni-O-C界面位点,用于在环境条件下从CO2和硝酸盐中高效和选择性地合成尿素。该催化剂是通过与聚多巴胺(PDA)螯合Ni2+离子,并将其锚定在石墨化碳氮(g-C3N4)上,形成坚固的杂化界面合成的。这种结构促进了CO2和NO3 -的同时活化,从而促进了C-N键的形成。在−1.3 V / RHE条件下,Ni-PDA@g-C3N4催化剂的尿素产率为1190.3 μg - h-1 mgcat-1,法拉第效率为18.03%,氮选择性为55.78%,且具有良好的长期稳定性。密度泛函理论(DFT)计算表明,Ni-O-C界面显著降低了关键中间体如*NHO和*COOH的能垒,从而加速了C-N耦合过程。这项工作强调了一种分子和界面设计策略,为从二氧化碳和含氮废物中可持续生产尿素提供了一条有前途的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


