Total Synthesis of Daphnepapytone A
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The total synthesis of sesquiterpenoid daphnepapytone A starting from (R)-carvone is reported. The carbocyclic framework of daphnepapytone A was formed by a one-pot Pauson–Khand/desilylative oxidation reaction and a [2 + 2] photocycloaddition of synthetic oleodaphnone. During the synthesis, it was discovered that wavelength-dependent irradiation of oleodaphnone resulted in intramolecular photometathesis to form a dicyclopentadienone. Late-stage oxidation and a highly stereo- and regioselective reduction completed the synthesis.
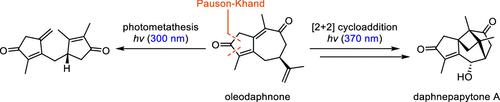
全合成麻黄酮A
本文报道了以(R)-香芹酮为起始原料合成倍半萜类麻黄酮A的方法。通过一锅Pauson-Khand /脱硅氧化反应和合成油苯甲酮的[2 + 2]光环加成形成了萘甲酮A的碳环骨架。在合成过程中,发现油达夫酮的波长依赖性照射导致分子内光度变化,形成双环戊二烯酮。后期氧化和高度立体和区域选择性还原完成了合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


