Nanomotor-Promoted In Situ Vaccination for Deep-Seated Cancer Immunotherapy
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In situ tumor vaccination, achieved through the induction of immunogenic cell death (ICD), has the potential to elicit robust antitumor immune responses. However, the limited penetration efficiency of drugs and the various immunosuppressive mechanisms present within cells hinder their development and clinical effectiveness. In this study, we propose a nanomotor (AHC-motor)-promoted in situ vaccination strategy for deep-seated cancer immunotherapy through immunomodulation and efficient extracellular matrix penetration. Specifically, the nanomotor consists of catalase-linked tetrasulfide bond-modified hollow mesoporous silica, with 5-aminolevulinic acid encapsulated within it. These AHC-motors exhibit active movement in the H2O2 environment and demonstrate enhanced penetration into deep tumor tissues, thereby improving the cellular uptake efficiency of the ICD inducer. Owing to the profound delivery of the ICD inducer, the fabrication of in situ vaccines and the activation of stimulator of interferon genes (STING) have been successfully accomplished. Importantly, these nanomotors, with adaptable preparation characteristics, are equipped with the capacity to promote the persulfidation of zinc finger proteins, resulting in an increased release of proinflammatory cytokines and facilitating the efficacy of in situ vaccine therapy. With its matrix penetration and immunomodulatory properties, the nanomotor may provide insights into the fabrication of in situ vaccines and the development of robust deep-seated immunotherapy.
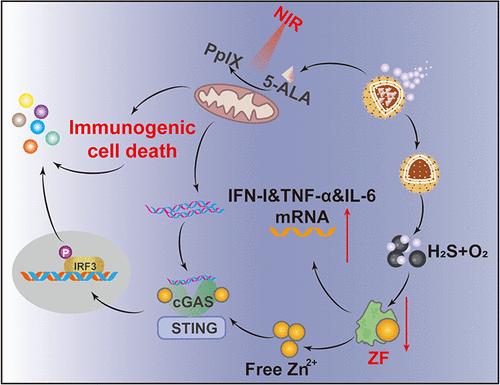
纳米马达促进的深部肿瘤免疫原位接种治疗
通过诱导免疫原性细胞死亡(ICD)实现的原位肿瘤疫苗接种有可能引发强大的抗肿瘤免疫反应。然而,药物有限的渗透效率和细胞内存在的各种免疫抑制机制阻碍了它们的发展和临床效果。在这项研究中,我们提出了一种纳米运动(AHC-motor)促进的原位疫苗接种策略,通过免疫调节和有效的细胞外基质渗透,用于深部肿瘤免疫治疗。具体来说,该纳米马达由过氧化氢酶连接的四硫键修饰的中空介孔二氧化硅组成,其内部包裹有5-氨基乙酰丙酸。这些ahc马达在H2O2环境中表现出积极的运动,并增强了对肿瘤组织深层的渗透,从而提高了ICD诱导剂的细胞摄取效率。由于ICD诱导剂的深入传递,原位疫苗的制造和干扰素基因刺激器(STING)的激活已经成功完成。重要的是,这些纳米马达具有适应性强的制备特性,具有促进锌指蛋白过硫化的能力,从而增加促炎细胞因子的释放,促进原位疫苗治疗的效果。由于其基质渗透和免疫调节特性,纳米马达可能为原位疫苗的制造和强大的深层免疫治疗的发展提供见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


