Prodrug-Doped ROS-Responsive Lipid Nanoparticle for Enhanced mRNA Delivery and Synergistic Anti-Tumor Therapy
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lipid nanoparticSle (LNP) represents the most advanced mRNA delivery platform for the development of effective cancer therapeutics. Nevertheless, conventional LNP-mRNA systems are hindered by inadequate endosomal release capability and a lack of synergistic integration with complementary therapeutic approaches. Herein, a reactive oxygen species (ROS)-responsive prodrug-doped LNP (LNP-Pro) that can enhance mRNA delivery efficacy and enable synergistic anti-tumor therapy is reported. A camptothecin (CPT)-based prodrug, conjugated to a PEG-containing block polymer via a ROS-cleavable linker, is successfully incorporated into clinically approved LNP formulations shown in a report. It shows that LNP-Pro can respond to the elevated ROS microenvironment in tumor cells, displaying superior delivery capacity in tumor cells relative to normal cells. Furthermore, LNP-Pro exhibits significantly enhanced mRNA delivery efficacy compared to conventional formulations. In vitro and in vivo studies confirm that LNP-Pro loaded with therapeutic mRNA synergizes with CPT chemotherapeutic to induce tumor cell apoptosis, achieving significant tumor growth inhibition in a murine model with excellent biocompatibility. Thus, this strategy integrates stimulus-responsive prodrugs with LNP systems, providing a versatile platform for mRNA-based cancer therapy.
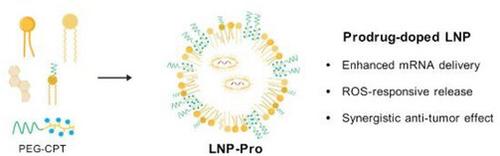
前药掺杂ros反应性脂质纳米颗粒增强mRNA传递和协同抗肿瘤治疗
脂质纳米颗粒(LNP)代表了最先进的mRNA传递平台,用于开发有效的癌症治疗药物。然而,传统的LNP-mRNA系统受到内体释放能力不足和缺乏与补充治疗方法的协同整合的阻碍。本文报道了一种活性氧(ROS)应答的前药掺杂LNP (LNP- pro),它可以提高mRNA的递送效率并实现协同抗肿瘤治疗。一份报告显示,一种喜树碱(CPT)为基础的前药,通过ros可切割的连接物与含peg的嵌段聚合物偶联,成功地纳入临床批准的LNP配方中。这表明LNP-Pro能够响应肿瘤细胞中升高的ROS微环境,在肿瘤细胞中表现出优于正常细胞的递送能力。此外,与常规配方相比,LNP-Pro表现出显著增强的mRNA递送效率。体外和体内研究证实,负载治疗mRNA的LNP-Pro与CPT化疗药物协同诱导肿瘤细胞凋亡,在具有良好生物相容性的小鼠模型中实现了显著的肿瘤生长抑制。因此,该策略将刺激反应性前药与LNP系统相结合,为基于mrna的癌症治疗提供了一个多功能平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


