Accelerated Regeneration of Senescent Bone Injury through Age- and Sex-Independent Macrophage Polarization
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nanomedicines offer broad therapeutic potential, but key host factors such as age and sex (now recognized as critical factors for efficacy) remain largely overlooked. Aging, which is characterized by systemic chronic inflammation and delayed tissue regeneration, poses significant medical and economic issues in aging societies. Older individuals exhibit impaired macrophage transition from an inflammatory M1 phenotype to an anti-inflammatory/pro-healing M2 phenotype, and this transition is a potential target for rejuvenating tissue repair. Existing therapeutic approaches, such as cytokines and biomaterial surface engineering, effectively promote M1-to-M2 polarization in young individuals, but their efficacy is markedly reduced in older individuals, and sex differences in therapeutic macrophage polarization remain largely unexplored. Here, we show that phosphatidylserine liposomes (PSLs) induced macrophage polarization independent of age (3–4 months old or >21 months old) and sex in mice. In addition, in vitro experiments confirmed that factors secreted by M1 macrophages inhibited osteoblast (OB) differentiation and enhanced osteoclast (OC) differentiation, with older macrophages from both sexes exerting more pronounced effects, while factors secreted by M2 macrophages had the opposite effect. Furthermore, in a critical-sized bone defect model in old mice, PSLs induced macrophage phenotype conversion, improved the balance between OB and OC differentiation, and eventually accelerated bone repair. These findings suggest that PSLs are a universal M2 macrophage inducer and offer a promising therapeutic strategy for restoring bone repair in older individuals as well as potentially promoting tissue regeneration in other organs.
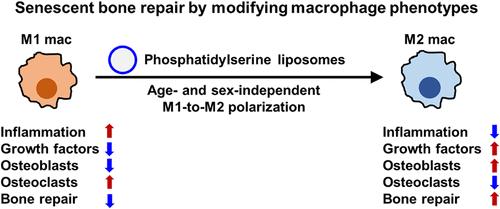
通过年龄和性别无关的巨噬细胞极化加速衰老骨损伤的再生
纳米药物提供了广泛的治疗潜力,但是关键的宿主因素,如年龄和性别(现在被认为是疗效的关键因素)在很大程度上仍然被忽视。衰老,其特点是全身性慢性炎症和延迟组织再生,在老龄化社会提出了重大的医疗和经济问题。老年人表现出巨噬细胞从炎性M1表型向抗炎/促愈合M2表型的转变受损,这种转变是恢复组织修复的潜在目标。现有的治疗方法,如细胞因子和生物材料表面工程,在年轻人中有效地促进了m1到m2的极化,但在老年人中其效果明显降低,并且治疗性巨噬细胞极化的性别差异在很大程度上仍未被探索。在这里,我们发现磷脂酰丝氨酸脂质体(PSLs)诱导巨噬细胞极化与年龄(3-4月龄或21月龄)和性别无关。此外,体外实验证实,M1巨噬细胞分泌的因子抑制成骨细胞(OB)分化,增强破骨细胞(OC)分化,且年龄较大的两性巨噬细胞的作用更为明显,而M2巨噬细胞分泌的因子则相反。此外,在老年小鼠临界尺寸骨缺损模型中,psl诱导巨噬细胞表型转化,改善OB和OC分化平衡,最终加速骨修复。这些发现表明,psl是一种通用的M2巨噬细胞诱导剂,为恢复老年人的骨修复以及潜在地促进其他器官的组织再生提供了一种有希望的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


