Low-coordination PtBi heterogeneous interface boosting the selective electrooxidation of ethylene glycol to value-added glycolic acid
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
The electrocatalytic oxidation of ethylene glycol (EG) into high-value chemicals like glycolic acid (GA) is a crucial step for upcycling waste plastics. However, catalyst deactivation and low selectivity pose significant challenges. This work presents the low-coordination PtBi nanosheets (LC-PtBi NSs), featuring a unique amorphous-crystalline heterostructure with a low coordination number of 2.3–2.5. They can exhibit exceptional mass activity (8.3 A mgPt−1) and stability (maintaining 88.7 % of initial activity after running for 3600 s) of the EG oxidation reaction (EGOR). They also achieve over 90 % apparent selectivity for EG-to-GA conversion at low potentials (<0.7 V vs. RHE) and even more than 100-h continuous electrolysis. Density functional theory (DFT) calculations reveal that the low-coordination PtBi heterogeneous interface is responsible for the high coverage of OHad species and weakened adsorption of carbonaceous intermediates on LC-PtBi NSs, thereby promoting the direct oxidation of C2 intermediates to GA. This work demonstrates a strategy of doping-mediated catalytic interface regulation and electron density rearrangement, offering insights for designing efficient Pt-based electrocatalysts toward selective oxidation of small molecules.
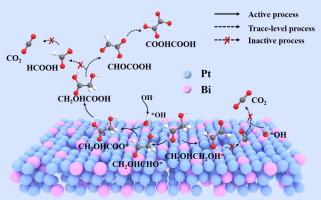
低配位PtBi非均相界面促进乙二醇选择性电氧化制高附加值乙醇酸
乙二醇(EG)的电催化氧化成高价值的化学品,如乙醇酸(GA)是废塑料升级回收的关键步骤。然而,催化剂失活和低选择性带来了重大挑战。本文提出了低配位PtBi纳米片(LC-PtBi NSs),具有独特的非晶异质结构,配位数低,为2.3-2.5。它们在EG氧化反应(EGOR)中表现出优异的质量活性(8.3 A mgPt−1)和稳定性(运行3600 s后仍保持88.7%的初始活性)。在低电位(<0.7 V vs. RHE)和超过100小时的连续电解下,它们也实现了超过90%的表观选择性eg - ga转换。密度泛函理论(DFT)计算表明,低配位的PtBi非均相界面是导致OHad物种高覆盖率和碳质中间体在LC-PtBi NSs上吸附减弱的原因,从而促进了C2中间体直接氧化成GA。这项工作证明了掺杂介导的催化界面调节和电子密度重排策略,为设计高效的基于pt的小分子选择性氧化电催化剂提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


