On-site electrocatalytic synthesis of hydrogen peroxide in potassium fertilizer solutions
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
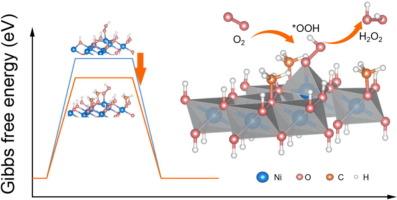
The two-electron electrochemical oxygen reduction reaction (ORR) affords an appealing alternative for on-site production of hydrogen peroxide (H2O2), which can fulfill the demands of various applications even at low concentrations. Under neutral or near-neutral conditions, the electrolyte environment capable of electrochemically synthesizing H2O2 exhibits diversity and holds vast potential for practical applications; however, the electrocatalytic performance is limited without desirable electrode materials. In this contribution, methoxylated nickel hydroxides were proposed for high-performance on-site H2O2 electrosynthesis in different potassium fertilizer solutions. The methoxylation compared to pristine Ni(OH)2 was demonstrated to optimize the electronic structure with favorable adsorption of reaction intermediates, obviously enhancing the activity and selectivity. In 0.10 M K2SO4 solution, H2O2 production ranged from 28.1 to 153.6 mg h−1 cm−2 at current densities of −50 to −250 mA cm−2, accompanied by Faradaic efficiency values exceeding 88.0 %. An integrated system was devised by combining fertilization, disinfection, and irrigation through the coupling of two-electron ORR with agricultural irrigation, utilizing nutrient solutions as the electrolyte for on-site H2O2 electrosynthesis. These findings afford a promising avenue for the practical application of 2e− ORR in neutral environments.
钾肥溶液中过氧化氢的现场电催化合成
双电子电化学氧还原反应(ORR)为现场生产过氧化氢(H2O2)提供了一种有吸引力的替代方案,即使在低浓度下也可以满足各种应用的需求。在中性或近中性条件下,能够电化学合成H2O2的电解质环境呈现多样性,具有巨大的实际应用潜力;然而,如果没有理想的电极材料,电催化性能就会受到限制。在这篇文章中,提出了甲氧基化氢氧镍在不同钾肥溶液中用于高性能的现场H2O2电合成。与原始Ni(OH)2相比,甲氧基化优化了电子结构,有利于反应中间体的吸附,明显提高了活性和选择性。在0.10 M K2SO4溶液中,当电流密度为- 50至- 250 mA cm - 2时,H2O2产率为28.1至153.6 mg h - 1 cm - 2,法拉第效率值超过88.0%。通过双电子ORR与农业灌溉的耦合,设计了施肥、消毒、灌溉相结合的综合系统,利用营养液作为电解液进行现场H2O2电合成。这些发现为2e - ORR在中性环境中的实际应用提供了一条有希望的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


