A Lipopolysaccharide-Directed Piezoelectric Sonosensitizer for Addressing Deep Periodontal Pocket Infections
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
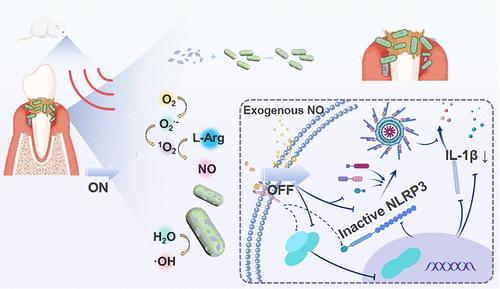
Complete removal of dental plaque biofilms and associated pathogens from deep periodontal pockets, coupled with subsequent inflammation resolution, constitutes a pivotal challenge in current periodontitis therapy. Here, a targeted bactericidal and gas-based anti-inflammatory strategy to regulate the periodontal microenvironment was established. The strategy aimed to eradicate deep-seated infections by modifying piezoelectric bimetallic Ti/Mn-MOFs nanosheets with phenylboronic acid (PBA) and l-arginine (l-arg). PBA mediated chemical targeting against periodontal pathogens via lipopolysaccharide (LPS) covalent coupling. Under ultrasound (US) conditions, the sonodynamic therapy (SDT) mediated by Ti/Mn-MOFs was employed to generate reactive oxygen species, thereby oxidizing l-arg and controllably releasing nitric oxide (NO). NO exhibits dual therapeutic functions: primarily through its bactericidal activity to disrupt biofilms and concomitantly via suppression of NLRP3 inflammasome activation to mitigate inflammatory responses. With its relatively long half-life and large diffusion radius, a synergistic effect characterized by a concentrated burst of bactericidal effect and sustained maintenance of anti-inflammatory concentration occurs under the regulation of the ultrasonic switch mechanism. Moreover, the 3d orbital electrons of Mn hybridized with the energy levels of the Ti–O framework, effectively narrowing the band gap of the Ti-MOFs and optimizing the SDT efficiency. This strategy offers an approach to break the vicious cycle of infection–inflammation in periodontitis.
一种用于治疗牙周深袋感染的脂多糖导向压电声敏剂
彻底清除深层牙周袋中的牙菌斑生物膜和相关病原体,再加上随后的炎症消退,构成了当前牙周炎治疗的关键挑战。本研究建立了一种靶向杀菌和基于气体的抗炎策略来调节牙周微环境。该策略旨在通过用苯硼酸(PBA)和l-精氨酸(l-arg)修饰压电双金属Ti/ mn - mof纳米片来根除深层感染。PBA介导的脂多糖共价偶联对牙周病原体的化学靶向作用。在超声(US)条件下,利用Ti/ mn - mof介导的声动力疗法(SDT)产生活性氧,从而氧化l-arg并可控释放一氧化氮(NO)。NO具有双重治疗功能:主要是通过其杀菌活性破坏生物膜,同时通过抑制NLRP3炎症小体激活来减轻炎症反应。其半衰期较长,扩散半径大,在超声开关机制的调节下,产生杀菌作用集中爆发,抗炎浓度持续维持的协同效应。此外,Mn的三维轨道电子与Ti-O骨架的能级杂化,有效地缩小了ti - mof的带隙,优化了SDT效率。这种策略提供了一种方法来打破牙周炎感染-炎症的恶性循环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


