Dual-Additive-Driven Boron-Enriched Interphases Stabilize Tetrahydrofuran-Based Electrolytes for High-Voltage Sodium-Ion Batteries
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The advancement of energy storage systems heavily relies on the development of high-performance electrolytes. Nonetheless, conventional electrolytes often struggle with interfacial stability, ionic conductivity, and electrochemical compatibility. Cyclic ether solvents, such as tetrahydrofuran (THF), despite their superior ionic conductivity and solvation kinetics, are plagued by intrinsic oxidative instability at high potentials (>4.0 V vs Na+/Na), which undermines their cycling durability with high-voltage cathodes. This study introduces a boron-rich dual-additive electrolyte that resolves the voltage-stability paradox of THF-based systems, by preferentially adsorbing onto the cathode and decomposing to form a robust, boron-enriched interfacial layer that precisely regulates interfacial chemistry and stabilizes high-voltage operation. Consequently, the optimal electrolyte significantly enhances the performance of high-voltage Na0.75Ni0.25Fe0.25Mn0.5O2 (NFM) cathodes, achieving 82.9% capacity retention after 150 cycles at a 4 V cut-off and delivering a remarkable rate capability of 80.4 mAh g−1 at 1 A g−1. Additionally, full cells with hard carbon anodes maintain 69.3% capacity over 150 cycles with an average Coulombic efficiency of 99.5%. This work establishes a general paradigm for designing oxidation-resistant electrolytes through targeted interface engineering, representing a fundamental leap toward high-voltage sodium-ion batteries.
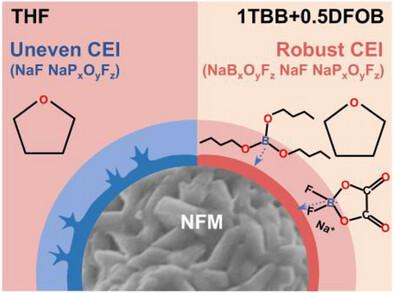
双添加剂驱动的富硼界面稳定高压钠离子电池用四氢呋喃电解质
储能系统的进步在很大程度上依赖于高性能电解质的发展。尽管如此,传统的电解质经常在界面稳定性、离子电导率和电化学相容性方面遇到困难。环醚溶剂,如四氢呋喃(THF),尽管具有优异的离子电导率和溶剂化动力学,但在高电位(>4.0 V vs Na+/Na)下存在固有的氧化不稳定性,这破坏了它们在高压阴极上的循环耐久性。本研究引入了一种富硼双添加剂电解质,通过优先吸附在阴极上并分解形成一个坚固的富硼界面层,精确调节界面化学并稳定高压操作,解决了基于thf的系统的电压稳定性悖论。结果表明,优化的电解液显著提高了高压Na0.75Ni0.25Fe0.25Mn0.5O2 (NFM)阴极的性能,在4 V截止电压下,150次循环后的容量保持率达到82.9%,在1 ag−1时的倍率容量达到80.4 mAh g−1。此外,使用硬碳阳极的全电池在150次循环中保持69.3%的容量,平均库仑效率为99.5%。这项工作为通过靶向界面工程设计抗氧化电解质建立了一个通用范例,代表了高压钠离子电池的根本性飞跃。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


