Exosomes derived from M1-macrophage promote enteric neuronal injury via MMP8-TGF-β pathway
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Enteric neuronal injury significantly contributes to gastrointestinal motility disorders. While macrophages are known to interact closely with enteric neurons and modulate intestinal homeostasis, their specific impact on enteric neuronal survival and injury remains poorly understood.Objective
This study aimed to explore the potential contribution of macrophage-derived exosomes to enteric neuronal injury and investigate the underlying mechanisms.Methods
We established a benzalkonium chloride (BAC)-induced enteric neuronal injury mouse model and assessed the effects of macrophage depletion on gastrointestinal motility and neuronal density. In vitro, enteric neurons were co-cultured with macrophages to examine the role of macrophage-derived exosomes in neuronal apoptosis. M1 macrophage-derived exosomes were isolated and characterized to elucidate potential mechanisms both in vitro and in vivo.Results
Macrophage depletion partially rescued gastrointestinal dysmotility and enteric neuronal injury in BAC-treated mice. In vitro studies confirmed that M1 macrophage-derived exosomes promoted neuronal apoptosis. We identified matrix metalloproteinase 8 (MMP8) as enriched in M1 macrophage-derived exosomes, promoting the activation of the TGF-β signaling pathway and inducing neuronal apoptosis. Exosomes derived from M1 macrophages exacerbated neuronal injury in BAC mice, while inhibiting MMP8 partially mitigated neuronal injury and restored intestinal function. Additionally, elevated serum exosomal MMP8 levels were identified as a potential marker for Hirschsprung’s disease, a typical gastrointestinal motility disorder.Conclusion
M1 macrophages induce enteric neuronal injury via exosomal MMP8-mediated TGF-β signaling, highlighting a potential therapeutic target for gastrointestinal motility disorders.
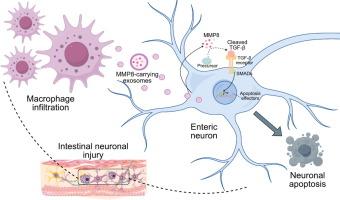
来自m1巨噬细胞的外泌体通过MMP8-TGF-β途径促进肠神经元损伤
肠神经损伤是引起胃肠运动障碍的重要因素。虽然已知巨噬细胞与肠神经元密切相互作用并调节肠道内稳态,但它们对肠神经元存活和损伤的具体影响仍知之甚少。目的探讨巨噬细胞来源的外泌体在肠神经元损伤中的潜在作用及其机制。方法建立苯扎氯铵(benzalkonium chloride, BAC)诱导的小鼠肠内神经元损伤模型,观察巨噬细胞耗竭对胃肠道运动和神经元密度的影响。在体外,将肠内神经元与巨噬细胞共培养,研究巨噬细胞来源的外泌体在神经元凋亡中的作用。M1巨噬细胞来源的外泌体被分离和表征,以阐明体外和体内的潜在机制。结果巨噬细胞耗竭可部分缓解bac处理小鼠的胃肠运动障碍和肠神经元损伤。体外研究证实,M1巨噬细胞来源的外泌体促进神经元凋亡。我们发现基质金属蛋白酶8 (MMP8)在M1巨噬细胞来源的外泌体中富集,促进TGF-β信号通路的激活,诱导神经元凋亡。来自M1巨噬细胞的外泌体加重了BAC小鼠的神经元损伤,而抑制MMP8可部分减轻神经元损伤并恢复肠道功能。此外,血清外泌体MMP8水平升高被确定为先天性巨结肠病(一种典型的胃肠运动障碍)的潜在标志物。结论m1巨噬细胞通过外泌体mmp8介导的TGF-β信号诱导肠神经元损伤,是胃肠道运动障碍的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


