Synthesis and characterization of Coumarin-Quinoline based NIR Probes for Viscosity: Mitochondria-Targeted Probe with Superior Performance in Autophagy and Liver Injury Imaging
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
Near-infrared (NIR) fluorescent probes with aggregation-induced emission (AIE) properties are highly desirable for biomedical imaging. Viscosity is a critical parameter reflecting microenvironment stability, and abnormal mitochondrial viscosity is linked to many diseases and cellular dysfunctions. Therefore, it is of important significance to develop new NIR fluorescent probes that can monitor the variations of mitochondrial viscosity accurately and in real time.Results
we developed three new NIR fluorescent probes (QI-1, QI-2, QI-3) based on a coumarin-quinoline structure with a donor-π-acceptor (D-π-A) design. These probes exhibit strong intramolecular charge transfer (ICT). Compared with the compounds QI-2 and QI-3, QI-1 showed outstanding performance, including excellent AIE behavior, a large Stokes shift, high quantum yield and high sensitivity to viscosity. Biological studies confirmed that QI-1 selectively localizes in mitochondria. This enables real-time monitoring of viscosity changes during cellular autophagy and in a mouse model of drug-induced (APAP) liver injury.Significance
This work presents a rational and effective design strategy for developing NIR fluorescent probes with AIE characteristics, specifically tailored for sensing microenvironmental viscosity. Probes based on this strategy allow for non-invasive, high-contrast visualization of pathophysiological processes across multiple biological scales, ranging from subcellular organelles to tissue microenvironments. This lays the foundation for advanced diagnostics and a deeper mechanistic understanding of mitochondrial dysfunction-related diseases.
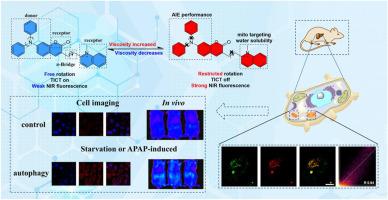
基于香豆素-喹啉的黏度近红外探针的合成与表征:线粒体靶向探针在自噬和肝损伤成像中的优越性能
具有聚集诱导发射(AIE)特性的近红外(NIR)荧光探针在生物医学成像中是非常理想的。黏度是反映微环境稳定性的关键参数,线粒体黏度异常与许多疾病和细胞功能障碍有关。因此,开发能够准确实时监测线粒体黏度变化的新型近红外荧光探针具有重要意义。结果基于香豆素-喹啉结构,采用供体-π-受体(D-π-A)结构设计了3种新型近红外荧光探针QI-1、QI-2、QI-3。这些探针表现出很强的分子内电荷转移(ICT)。与化合物QI-2和QI-3相比,QI-1表现出优异的AIE行为,Stokes位移大,量子产率高,对粘度的敏感性高。生物学研究证实QI-1选择性定位于线粒体。这使得可以实时监测细胞自噬期间和药物诱导(APAP)肝损伤小鼠模型中的粘度变化。本工作提出了一种合理有效的设计策略,用于开发具有AIE特性的近红外荧光探针,专门用于检测微环境粘度。基于这种策略的探针可以实现跨多个生物尺度(从亚细胞细胞器到组织微环境)的病理生理过程的非侵入性、高对比度可视化。这为线粒体功能障碍相关疾病的高级诊断和更深层次的机制理解奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


