Cobalt-embedded carbon nanotube-bridged hollow carbon cubes enabling electro n/ion co-transport and catalytic conversion in lithium-sulfur batteries
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
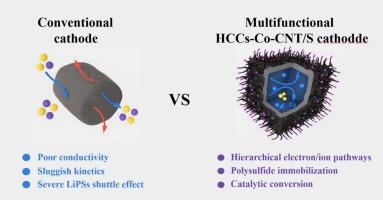
Lithium‑sulfur (Li−S) batteries have attracted tremendous attention as next-generation energy storage systems due to their exceptional theoretical energy density (2600 Wh kg−1). Despite their great promise, the large-scale application of Li−S batteries is still hindered by critical challenges, particularly the lithium polysulfide shuttle phenomenon and sluggish kinetics during sulfur-based redox reactions. This research introduces an innovative cathode architecture that consists of hollow carbon cubes integrated with carbon nanotubes and cobalt nanoparticles (HCCs-Co-CNTs), aiming to mitigate the previously discussed issues. The HCCs-Co-CNTs possess structure featuring cobalt-embedded carbon nanotubes to bridge hollow carbon cubes, forming a multi-scale and interconnected structure. The design enables synergistic enhancement of Li−S battery performances through several mechanisms: The hollow carbon cubes provide a continuous conductive framework for efficient electron transport; The interconnected carbon structure improves electrical conductivity across the cathode; Carbon nanotubes (CNTs) directly grown on the surfaces of the cubes establish continuous conductive networks, resulting in a three-dimensional ion transport network to facilitate lithium-ion diffusion. Meanwhile, cobalt nanoparticles embedded within the nanotubes act as catalytic centers, improving the chemical interaction with polysulfides and facilitating their catalytic transformation, enabling coordinated regulation of electrons, ions, and reactants. This synergistic design enables the HCCs-Co-CNTs/S cathode to deliver an initial discharge capacity of 1216 mAh g−1 at 0.2C with 83 % capacity retention after 200 cycles. Even at a high rate of 4C, it maintains a capacity of 734 mAh g−1, demonstrating excellent rate capability and cycling stability. This study provides an effective approach to mitigate the LiPSs shuttle effect and contributes to the rational development of sulfur host architectures.
嵌入钴的碳纳米管桥接的空心碳立方体,可在锂硫电池中实现电子/离子共输运和催化转化
锂硫(Li−S)电池作为下一代储能系统,由于其卓越的理论能量密度(2600 Wh kg−1)而引起了极大的关注。尽管前景广阔,但锂- S电池的大规模应用仍然受到关键挑战的阻碍,特别是多硫化锂穿梭现象和硫基氧化还原反应过程中的缓慢动力学。本研究介绍了一种创新的阴极结构,该结构由碳纳米管和钴纳米颗粒(HCCs-Co-CNTs)集成的空心碳立方体组成,旨在缓解先前讨论的问题。HCCs-Co-CNTs具有嵌钴碳纳米管的结构,可桥接空心碳立方体,形成多尺度互联结构。该设计能够通过几种机制协同增强Li−S电池的性能:中空碳立方体为有效的电子传递提供了连续的导电框架;相互连接的碳结构提高了阴极的导电性;碳纳米管(CNTs)直接生长在立方体表面,建立连续的导电网络,形成三维离子传输网络,促进锂离子扩散。同时,嵌入在纳米管中的钴纳米颗粒作为催化中心,改善了与多硫化物的化学相互作用,促进了它们的催化转化,实现了电子、离子和反应物的协调调节。这种协同设计使HCCs-Co-CNTs/S阴极在0.2C下提供1216 mAh g−1的初始放电容量,在200次 循环后容量保持率为83% %。即使在4C的高倍率下,它也保持734 mAh g−1的容量,表现出出色的倍率能力和循环稳定性。该研究为减轻LiPSs的穿梭效应提供了有效途径,有助于硫宿主结构的合理发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


