N-Alkyl Sulfamates as a New Class of nsP2 Cysteine Protease Inhibitors with Broad-Spectrum Antialphaviral Activity
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
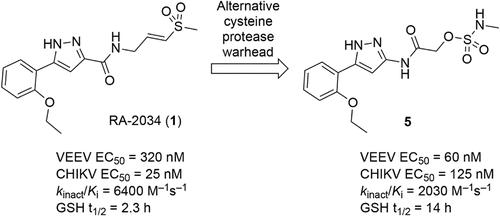
The emergence of mosquito-borne alphaviruses that cause chronic arthritis or encephalitis underscores the urgent need for broad-spectrum antiviral therapeutics. The viral nsP2 cysteine protease, which is essential for alphavirus replication, is a promising antiviral target. Vinyl sulfone covalent inhibitors potently inhibit nsP2 protease but suffer from glutathione reactivity and species-dependent systemic clearance catalyzed by glutathione S-transferase. To address these liabilities, we explored alternative electrophilic warheads and identified acetamide inhibitors bearing N-alkyl sulfamate warheads with improved biochemical and antiviral profiles. 2-((5-(2-Ethoxyphenyl)-1H-pyrazol-3-yl)amino)-2-oxoethyl methylsulfamate emerged as a lead compound with potency against New and Old World alphaviruses, low GSH reactivity, and proteome-wide selectivity. Despite its promising antialphaviral activity, 2-((5-(2-ethoxyphenyl)-1H-pyrazol-3-yl)amino)-2-oxoethyl methylsulfamate exhibited rapid clearance due to hepatic glucuronidation. Structure–activity studies revealed modifications that improve metabolic stability while retaining antiviral activity. These findings introduce sulfamate acetamides as a new class of covalent nsP2 protease inhibitors and direct-acting pan-alphavirus drugs.
具有广谱抗病毒活性的新型nsP2半胱氨酸蛋白酶抑制剂n -烷基氨基磺酸盐
引起慢性关节炎或脑炎的蚊媒甲病毒的出现强调了对广谱抗病毒治疗的迫切需要。病毒nsP2半胱氨酸蛋白酶是甲病毒复制所必需的,是一个很有前途的抗病毒靶点。乙烯基砜共价抑制剂能有效抑制nsP2蛋白酶,但具有谷胱甘肽反应性和谷胱甘肽s-转移酶催化的物种依赖性全身清除。为了解决这些问题,我们探索了可替代的亲电弹头,并确定了带有n -烷基磺酸盐弹头的乙酰胺抑制剂,这些弹头具有改善的生化和抗病毒特性。2-((5-(2-乙氧基苯基)- 1h -吡唑-3-基)氨基)-2-氧乙基甲基磺胺酸作为一种先导化合物出现,具有抗新、旧大陆甲病毒的效力、低谷胱甘肽反应性和蛋白质组选择性。尽管2-((5-(2-乙氧基苯基)- 1h -吡唑-3-基)氨基)-2-氧乙基甲基磺胺酸具有很好的抗病毒活性,但由于肝脏葡萄糖醛酸化作用,它表现出快速清除的能力。结构-活性研究揭示了在保持抗病毒活性的同时改善代谢稳定性的修饰。这些发现介绍了氨基甲酸乙酯作为一类新的共价nsP2蛋白酶抑制剂和直接作用的泛甲病毒药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


