Regulating nitrogen speciation via temperature/Fe2O3 synergy in freshwater sludge for enhanced peracetic acid (PAA) activation and emerging contaminants removal
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
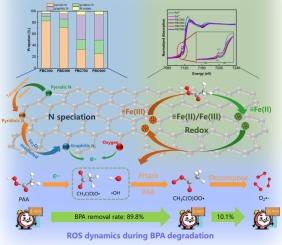
Rational regulation of transition metal sites and surface N species in catalysts is crucial for boosting peracetic acid (PAA) activation and its application in degrading emerging contaminants in water. This study presents a simple strategy for regulating surface Fe(II/III) sites and N speciation in freshwater sludge-derived biochar (FBC) by modulating pyrolysis temperature. As the temperature increased from 300 °C to 900 °C, Fe(III)-driven conversion of pyridinic N to graphitic N increased significantly (6.07 % to 45.00 %), concurrently elevating Fe(II) content. Both synergistically enhanced PAA activation. FBC700 outperformed FBC900 in PAA activation, attributed to its significantly higher specific surface area (65.93 m2/g vs. 22.03 m2/g) despite comparable graphitic N (43.19 % vs. 45.00 %) and Fe(II) content (15.86 % vs. 16.68 %). The dynamic evolution of reactive oxygen species was elucidated: Initial PAA adsorption on FBC700 and activation by electron-rich functional groups and ≡Fe(II) generated CH3C(O)O• and •OH, attacking PAA. Simultaneously, ≡Fe(III) reduction by PAA produced CH3C(O)OO•, while CH3C(O)O• decomposed into CH3• or reacted to form CH3C(O)OO•, ultimately yielding O2•−. In addition, the direct electron transfer pathway also contributes to BPA degradation by generated PAA* on FBC700 surface. FBC700/PAA shows great practical potential, evidenced by wide adaptability in water bodies and excellent regeneration. This study provides a novel and facile method for regulating active sites for PAA activation and reutilizing bulk freshwater sludge.
通过温度/Fe2O3协同作用调节淡水污泥中氮的形态,以增强过氧乙酸(PAA)的活化和新兴污染物的去除
催化剂中过渡金属位和表面N的合理调控是提高过氧乙酸(PAA)活性及其在水中新出现污染物降解中的应用的关键。本研究提出了一种通过调节热解温度来调节淡水污泥生物炭(FBC)表面Fe(II/III)位点和N形态的简单策略。当温度从300 °C升高到900 °C时,Fe(III)驱动的吡啶N向石墨N的转化率显著提高(6.07 %至45.00 %),同时Fe(II)含量升高。两者协同增强了PAA的活化。FBC700在PAA活化方面优于FBC900,这是由于其比表面积(65.93 m2/g vs. 22.03 m2/g)明显更高,尽管石墨N(43.19 % vs. 45.00 %)和Fe(II)含量(15.86 % vs. 16.68 %)相当。阐明了活性氧的动态演化:初始PAA在FBC700上吸附,富电子官能团和≡Fe(II)活化生成CH3C(O)O•和•OH,攻击PAA。同时,≡Fe(III)被PAA还原生成CH3C(O)OO•,而CH3C(O)O•分解为CH3•或反应生成CH3C(O)OO•,最终生成O2•−。此外,直接电子转移途径也有助于通过在FBC700表面生成PAA*来降解BPA。FBC700/PAA具有广泛的水体适应性和优异的再生性能,具有很大的应用潜力。本研究为PAA活化活性位点的调控和散装淡水污泥的再利用提供了一种新颖易行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


