Effects of nonsense genetic variants on OATP1B1 expression and transport activity in vitro
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Organic anion transporting polypeptide 1B1 (OATP1B1, encoded by SLCO1B1) is an influx transporter expressed in the basolateral membrane of hepatocytes, playing a key role in the hepatic uptake and clearance of various drugs and endogenous compounds from the plasma. Single nucleotide variants in the SLCO1B1 gene have been shown to alter plasma drug concentration by affecting transporter activity. Furthermore, several variants leading to premature termination of translation have been observed, together with loss-of-function SLCO1B3 variants, in patients with Rotor syndrome. In this study, we investigated whether selected nonsense variations (c.757C>T, c.1738C>T, c.1877T>A, c.1905–1909del, c.1925–1929del, c.1929–1932del, c.1928T>G, c.1968dup, c.1976C>G) affect the transport activity and membrane expression of OATP1B1. HEK293 cells were transduced with baculovirus constructs carrying either a variant or reference SLCO1B1 sequence and the uptake of 2′,7′-dichlorofluorescein or rosuvastatin was measured. Protein abundance was measured using a quantitative targeted absolute proteomics approach. Compared to reference OATP1B1, the c.1968dup and c.1976C>G variants, located near the C-terminus, retained 30–40 % of transport activity. In contrast, the remaining variants, located before or in the last transmembrane helix, resulted in complete loss of OATP1B1 function. Furthermore, all variants reduced OATP1B1 expression in the cell membranes. These results suggest that most nonsense variants result in a loss of OATP1B1 activity, but those located in the C-terminal loop may retain some activity. Moreover, carriers of the studied variants may exhibit increased plasma concentrations of OATP1B1 substrates.
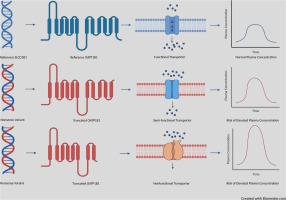
无义基因变异对体外OATP1B1表达和转运活性的影响
有机阴离子转运多肽1B1 (OATP1B1,由SLCO1B1编码)是一种表达于肝细胞基底外膜的内流转运体,在肝脏对血浆中各种药物和内源性化合物的摄取和清除中起关键作用。SLCO1B1基因的单核苷酸变异已被证明通过影响转运蛋白活性来改变血浆药物浓度。此外,在转子综合征患者中观察到几种导致翻译过早终止的变异,以及功能丧失的SLCO1B3变异。在这项研究中,我们研究了选择的无意义变异(c.757C>T, c.1738C>T, c.1877T>A, c.1905-1909del, c.1925-1929del, c.1929-1932del, c.1928T>G, c.1968dup, c.1976C>G)是否影响OATP1B1的转运活性和膜表达。用携带变异或参考SLCO1B1序列的杆状病毒构建体转导HEK293细胞,并测量2',7'-二氯荧光素或瑞舒伐他汀的摄取。使用定量靶向绝对蛋白质组学方法测量蛋白质丰度。与参考OATP1B1相比,位于c端附近的c.1968dup和c.1976C >g变体保留了30-40%的运输活性。相反,其余的变异,位于最后一个跨膜螺旋之前或之后,导致OATP1B1功能的完全丧失。此外,所有变异都降低了细胞膜中OATP1B1的表达。这些结果表明,大多数无义变异导致OATP1B1活性的丧失,但那些位于c端环的变异可能保留一些活性。此外,研究变异的携带者可能表现出OATP1B1底物的血浆浓度增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


