Cascade catalysis-based signal amplification for colorimetric detection of acetylcholinesterase and its inhibitors
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
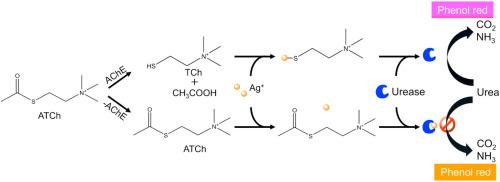
Cascade catalysis represents a fundamental physiological process and serves as a highly effective strategy for signal amplification in biosensing. Acetylcholinesterase (AChE) is a pivotal enzyme in neurological function, acting not only as a critical biomarker for neurodegenerative diseases but also as a primary target for pharmaceuticals and pesticides. Consequently, the detection of AChE activity and the screening of its inhibitors are essential for clinical diagnostics, drug development, and environmental monitoring. Reliable methods for trace-level AChE analysis remain a critical challenge that needs to be addressed promptlyResults
We reported a novel AChE-urease cascade catalysis amplification strategy for the colorimetric detection of AChE activity and its inhibitors. In this system, AChE catalyzes the hydrolysis of thioacetylcholine chloride (ATCh) to produce thiocholine (TCh). TCh then binds to Ag+ via its thiol group, thereby alleviating the inhibitory effect of Ag+ on urease activity. Urease with high activity catalyzes urea hydrolysis, leading to a rise in pH, which is monitored using the pH indicator phenol red. Leveraging cascade catalysis, this method achieves highly sensitive detection of AChE with a limit of detection (LOD) as low as 0.0116 mU/mL. The feasibility for inhibitor screening was validated using dipterex and berberine as model inhibitors, yielding IC50 values of 28 ng/mL and 14.4 μM, and LODs of 0.394 ng/mL and 0.23 μM, respectively.Significance
These results present a novel signal amplification strategy relying on enzyme cascade catalysis. This approach boasts advantages like simplicity, high sensitivity, and low cost. It not only holds promising applications in detecting AChE and its inhibitors for agriculture, medicine, and biosensing but also may extend to other enzymes that mediate thiol transformations.
基于级联催化的信号放大比色检测乙酰胆碱酯酶及其抑制剂
级联催化是一种基本的生理过程,是生物传感中信号放大的一种高效策略。乙酰胆碱酯酶(AChE)是神经功能的关键酶,不仅是神经退行性疾病的重要生物标志物,也是药物和农药的主要靶点。因此,乙酰胆碱酯酶活性的检测及其抑制剂的筛选对临床诊断、药物开发和环境监测至关重要。结果我们报道了一种新的乙酰胆碱酯酶脲酶级联催化扩增策略,用于比色检测乙酰胆碱酯酶活性及其抑制剂。在该体系中,乙酰胆碱酯酶催化硫代乙酰胆碱氯(ATCh)水解生成硫代胆碱(TCh)。然后TCh通过其巯基与Ag+结合,从而减轻Ag+对脲酶活性的抑制作用。高活性脲酶催化尿素水解,导致pH升高,用pH指示剂酚红监测。利用级联催化,该方法实现了AChE的高灵敏度检测,检出限(LOD)低至0.0116 mU/mL。以双百虫和小檗碱为模型抑制剂,验证了抑制剂筛选的可行性,IC50分别为28 ng/mL和14.4 μM, lod分别为0.394 ng/mL和0.23 μM。这些结果提出了一种依赖酶级联催化的新型信号放大策略。该方法具有简单、灵敏度高、成本低等优点。它不仅在检测AChE及其抑制剂的农业、医学和生物传感方面具有广阔的应用前景,而且还可以扩展到介导硫醇转化的其他酶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


