Asymmetric nano-disordering with favored charge and reaction kinetics to boost CO2 photoreduction with high stability
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
CO2 photoreduction is a promising approach to address global energy crisis and achieve carbon neutralization, yet the activity and stability are still far from satisfactory because of sluggish charge and reaction kinetics. Herein, we constructed a nanoscale asymmetric structure by integrating amorphous and crystalline nano-domains on two-dimensional ultrathin TiO2 nanosheets, which exhibits a boosted CO2 reduction activity with a CO production rate of 55.61 μmol g−1 h−1, and a prolonged stability with 8-cycle tests of 48 h in total, significantly outperforming its symmetric counterparts. In situ light-assisted surface photovoltage microscopy under CO2 atmosphere and density functional theory calculations suggest that the asymmetric Fermi levels of the ordered and disordered nano-domains lead to electron density gradient along the nano-interfaces, enhancing the separation and migration of photogenerated charge carriers. Also, due to the asymmetric electron acceptor-donor capability of the two domains, the disordered domain serves as active sites for CO2 reduction, while the ordered domain for water oxidation. The inverse electron flow directions on the two domains help to close the loop of electron transfer between redox sites, accelerating surface reaction kinetics. The simultaneous improvements of charge and reaction kinetics contribute to the excellent performance of the asymmetric catalyst, providing a new paradigm for designing photocatalysts through nanoscale disorder engineering.
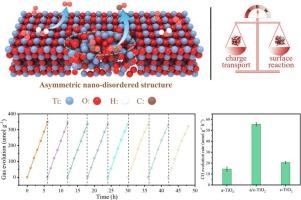
具有有利电荷的不对称纳米无序化和反应动力学促进高稳定性CO2光还原
CO2光还原是解决全球能源危机和实现碳中和的一种很有前景的方法,但由于电荷和反应动力学缓慢,其活性和稳定性仍远不能令人满意。本文通过在二维超薄TiO2纳米片上集成非晶和非晶纳米结构域,构建了纳米尺度的非对称结构,其CO产率为55.61 μmol g−1 h−1,并且具有较长的稳定性,8次循环测试共48 h,明显优于对称结构。CO2气氛下的原位光辅助表面光电压显微镜和密度泛函理论计算表明,有序和无序纳米畴的非对称费米能级导致沿纳米界面的电子密度梯度,增强了光生载流子的分离和迁移。此外,由于这两个结构域具有不对称的电子受体-供体能力,无序结构域充当CO2还原的活性位点,而有序结构域充当水氧化的活性位点。这两个结构域上的反向电子流方向有助于关闭氧化还原位点之间的电子转移循环,加速表面反应动力学。电荷动力学和反应动力学的同步改善使得不对称催化剂具有优异的性能,为通过纳米尺度无序工程设计光催化剂提供了新的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


