Amoebae contribute to the diversity and fate of antibiotic resistance genes in drinking water system
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Free-living amoebae represent a significant eukaryotic group that thrives in drinking water systems, posing considerable risks to water quality due to their inherent pathogenicity and associations with various microorganisms. However, the symbiotic microbial profiles of different amoeba species and the impact of amoeba-bacteria interactions on the antibiotic resistome within drinking water systems remain poorly understood. In this study, we obtained 24 amoeba isolates from tap water, encompassing diverse phyla within the amoeba lineage. Through metagenome sequencing, we uncovered variations in symbiotic microbiome composition across different amoeba species and strains. Notably, amoebae acted as vectors for human pathogens, including bacteria and viruses. The majority of symbionts carried multiple antibiotic-resistance genes and virulence factors. Furthermore, dominant symbiotic species could be cultured independently, underscoring the critical role of amoebae in preserving and transmitting antibiotic-resistant opportunistic pathogens in drinking water systems. Disinfection experiments demonstrated highly diverse viability of amoebae and their protective capabilities for symbionts against chlorine disinfection. Our findings expand the germplasm bank for amoebae and symbiotic bacteria derived from tap water and emphasize the necessity for further research on amoeba-bacteria symbiosis to ensure drinking water quality and public health safety
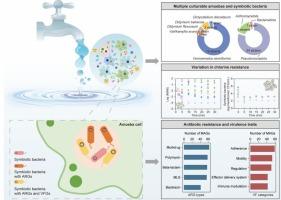
变形虫对饮用水系统中抗生素耐药基因的多样性和命运起着重要作用
自由生活的变形虫是一种重要的真核生物,在饮用水系统中茁壮成长,由于其固有的致病性和与各种微生物的关联,对水质构成相当大的风险。然而,不同阿米巴原虫种类的共生微生物特征以及阿米巴原虫-细菌相互作用对饮用水系统中抗生素抗性组的影响仍然知之甚少。在这项研究中,我们从自来水中分离得到24株变形虫,包括变形虫谱系中的不同门。通过宏基因组测序,我们发现了不同变形虫物种和菌株之间共生微生物组组成的差异。值得注意的是,变形虫是人类病原体(包括细菌和病毒)的载体。大多数共生体携带多种耐药基因和毒力因子。此外,优势共生物种可以独立培养,强调阿米巴在饮用水系统中保存和传播耐抗生素机会性病原体的关键作用。消毒实验表明变形虫具有高度多样化的生存能力及其对共生体对氯消毒的保护能力。本研究结果扩大了自来水中阿米巴细菌和共生细菌的种质资源库,强调了进一步研究阿米巴细菌与共生细菌的必要性,以确保饮用水质量和公共卫生安全
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


