1,2-Aminosulfonylation of Alkenes via a Photoexcited Cu(I)-Substrate Complex: A Three-Component Coupling Approach
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We report a Cu(I)-photocatalyzed three-component coupling process to achieve regioselective, vicinal 1,2-aminosulfonylations of alkenes. The critical aspect of this methodology is found in the utilization of in situ generated photoactive Cu(I)-amine substrate complexes, which facilitate the formal oxidation of Cu(I) to Cu(III) in a stepwise manner. In this way, the conceivable two-component chlorosulfonylation of alkenes is effectively suppressed, allowing the combination of styrene derivatives, sulfonyl chlorides, and amines. Furthermore, this protocol can be used to facilitate late-stage functionalization, thereby enabling the diversification of biomolecules.
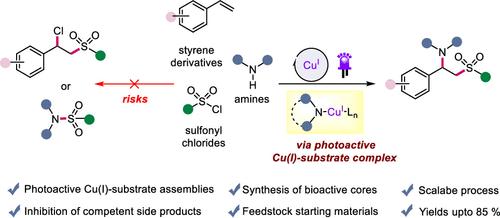
光激发Cu(I)-底物配合物的1,2-氨基磺酰化反应:三组分耦合方法
我们报道了Cu(I)光催化的三组分偶联过程,以实现区域选择性,邻域1,2-氨基磺酰化的烯烃。该方法的关键方面是利用原位生成的光活性Cu(I)-胺底物配合物,促进Cu(I)逐步氧化为Cu(III)。通过这种方式,可以想象的烯烃双组分氯磺化反应被有效抑制,从而允许苯乙烯衍生物、磺酰氯和胺的结合。此外,该协议可用于促进后期功能化,从而实现生物分子的多样化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


