Simultaneous Influence of Electrolytes and pH on Solubility and Distribution Coefficients of Ionizable Pharmaceuticals
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The influence of electrolytes (NaCl or KCl) and pH on solubility and octanol–water distribution was investigated for the active pharmaceutical ingredients (APIs) naproxen, ibuprofen, and lidocaine. The solubilities and partition coefficients were measured by UV/vis spectroscopy. The thermodynamically consistent framework for applying ePC-SAFT to ionizable APIs developed in previous work was expanded to account for electrolytes. Their influence on pH-dependent solubilities was fully predicted in quantitative agreement with experimental data. The octanol–water distribution coefficients of the APIs were obtained in good agreement with the experimental findings. This is noteworthy, as all model parameters were taken from the literature or determined from data on the pure components or binary subsystems. The model revealed that electrolytes decrease the degree of ionization of the APIs, which affects both their solubility and octanol–water distribution coefficients. Compared to electrolyte-free systems, the API solubility decreased by 30%, while their distribution coefficients increased 3-fold per mole of electrolyte.
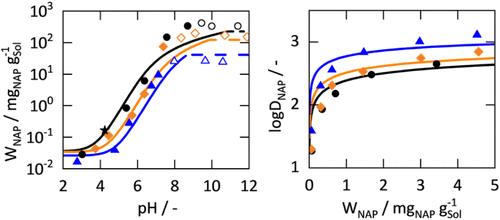
电解质和pH对可电离药物溶解度和分布系数的同时影响
考察了电解质(NaCl或KCl)和pH对萘普生、布洛芬和利多卡因的溶解度和辛醇-水分布的影响。用紫外/可见光谱法测定了溶解度和分配系数。将ePC-SAFT应用于先前工作中开发的可电离api的热力学一致框架扩展到考虑电解质。它们对ph依赖性溶解度的影响在定量上与实验数据完全一致。得到了原料药的辛醇-水分布系数,与实验结果吻合较好。这是值得注意的,因为所有模型参数都取自文献或从纯组件或二元子系统的数据中确定。模型表明,电解质降低了原料药的电离度,从而影响了其溶解度和辛醇-水分布系数。与无电解质体系相比,原料药的溶解度降低了30%,而每摩尔电解质的分布系数提高了3倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


