Pyrazine Formation from Saccharides and Alanine under Hydrothermal Conditions
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The reaction products, mechanisms, and kinetics of alanine reacting with saccharides (specifically cellobiose and glucose) under hydrothermal conditions (220–280 °C; 0.5–3 s) have been studied to understand the formation of nitrogen species in biofuels. The nitrogenous species found in the product mixture, including ammonia, ethylamine, and pyrazines, account for 50 to 75% of the total nitrogen balance. The carbonaceous products in the mixture are fructose, glyceraldehyde, dihydroxyacetone, formic acid, acetic acid, and furans. Our kinetic analysis shows that the nature of saccharides and amino acids plays an important role in the Maillard reaction rate. The Maillard reaction involving saccharides and amino acids with larger structures (such as cellobiose and alanine), proceeds more slowly than reactions involving simpler molecules (such as glucose and ammonium). For instance, at 280 °C, we deduce the rate constants of cellobiose–alanine and glucose–alanine = 0.011 and 0.035 s–1 mM–1, respectively. We proposed that when cellobiose and alanine are subjected to hydrothermal conditions, the primary pathway for pyrazine formation occurs as cellobiose decomposes into glucose, which then reacts with alanine following the Maillard reaction scheme.
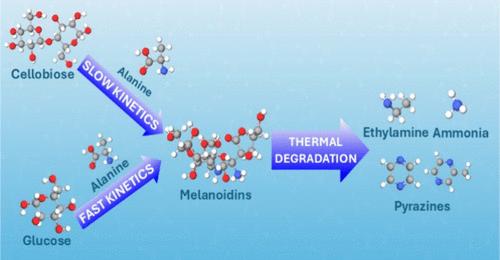
水热条件下糖和丙氨酸生成吡嗪的研究
研究了丙氨酸与糖类(特别是纤维素二糖和葡萄糖)在水热条件下(220-280°C; 0.5-3 s)的反应产物、机理和动力学,以了解生物燃料中氮种的形成。在产品混合物中发现的含氮物种,包括氨、乙胺和吡嗪,占总氮平衡的50 - 75%。混合物中的碳质产物是果糖、甘油醛、二羟基丙酮、甲酸、乙酸和呋喃。动力学分析表明,糖和氨基酸的性质对美拉德反应速率有重要影响。美拉德反应涉及结构较大的糖和氨基酸(如纤维素二糖和丙氨酸),比涉及简单分子(如葡萄糖和铵)的反应进行得慢。例如,在280°C下,我们推导出纤维素二糖-丙氨酸和葡萄糖-丙氨酸的速率常数分别为0.011和0.035 s-1 mM-1。我们提出,当纤维素二糖和丙氨酸处于水热条件下时,吡嗪形成的主要途径是纤维素二糖分解成葡萄糖,然后根据美拉德反应方案与丙氨酸反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


