Design, performance, and charge transfer insights into step-scheme zinc hydroxystannate/titanium dioxide heterostructures for enhanced photocatalytic oxidation of gaseous benzene
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
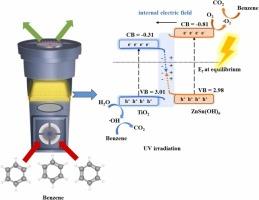
The design of a photocatalyst is crucial for the efficient photocatalytic oxidation (PCO) of indoor air pollutants like volatile organic compounds (VOCs). A highly effective strategy for enhancing photocatalytic activity is the construction of step (S)-scheme heterojunctions, which are engineered by coupling semiconductors such as n-type zinc hydroxystannate (ZnSn(OH)6, ZS) with n-type titanium dioxide (TiO2, T). The potential of the formed ZST photocatalyst is explored for the PCO of 1 ppm benzene under 1 W ultraviolet (UV) irradiation. It achieves a clean air delivery rate of 1.71 L min-1 with a 10% removal efficiency rate of 21.9 µmol g-1 h-1 and a mass-normalized apparent quantum yield of 6.08 ×10-4 molecule photon-1 g-1. The PCO activity of ZST is further assessed under several process variables. In situ DRIFTS analysis indicates that benzene mineralization proceeds through phenolate, acetate, maleate, and methylene reaction intermediates. Theoretical analyses (charge density difference and electron localization function) confirm an interfacial S-scheme electron transfer from ZnSn(OH)6 to TiO2. This mechanism effectively separates highly reactive carriers while promoting recombination of less active species for efficient benzene mineralization to carbon dioxide (CO2) and water (H2O). These findings provide valuable insights for designing high-performance catalytic systems against robust aromatic hydrocarbons.
设计,性能和电荷转移洞察步进方案羟基锡酸锌/二氧化钛异质结构增强光催化氧化气体苯
光催化剂的设计对于有效光催化氧化室内空气污染物(如挥发性有机化合物(VOCs))至关重要。通过将n型羟基锡酸锌(ZnSn(OH)6, ZS)等半导体与n型二氧化钛(TiO2, T)偶联,构建阶梯(S)方案异质结是提高光催化活性的一种高效策略。研究了所制ZST光催化剂在1w紫外光照射下,苯浓度为1ppm时的催化性能。洁净空气输送率为1.71 L min-1, 10%的去除率为21.9µmol g-1 h-1,质量归一化表观量子产率为6.08 ×10-4分子光子-1 g-1。在几个过程变量下进一步评估了ZST的PCO活性。原位漂移分析表明,苯矿化通过酚酸盐、醋酸盐、马来酸盐和亚甲基反应中间体进行。理论分析(电荷密度差和电子局域化函数)证实了ZnSn(OH)6向TiO2的界面S-scheme电子转移。该机制有效地分离了高活性载体,同时促进了活性较低的物质的重组,从而有效地将苯矿化为二氧化碳和水。这些发现为设计高性能的芳烃催化体系提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


