High-Throughput Electron Transfer in Inorganic–Organic Interfacial Electric Field Enabling Selective CO2 Photoreduction
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The efficiency of photocatalytic CO2 reduction has long been limited by the competing H2 evolution reaction. In this study, we present an innovative strategy for boosting high-throughput electron transfer to suppress H2 evolution, thereby enhancing CO2 reduction. By employing CdS and cobalt bipyridine as a model, we engineered the surface of CdS to create an electric field at the inorganic–organic interface. Through in situ and transient spectroscopy techniques, we discovered that CdS functionalized with ─COOH groups demonstrates remarkable noncovalent interactions and improved charge transfer capabilities compared to those functionalized with ─NH2 groups. The fast delivery of electrons on cobalt bipyridine facilitates the adsorbed CO2 to participate in the proton-electron coupling reaction, rather than allowing adsorbed protons to accept electrons directly. Consequently, the established CdS-COOH/Co(II)-bpy system achieved a CO production rate of 2.523 mmol g−1 h−1 with a selectivity of 96.3%. This research presents an approach for creating efficient charge transport interfaces and provides a comprehensive strategy for designing high-performance photocatalytic CO2 reduction systems that effectively counteract the challenges posed by competing H2 evolution reactions.
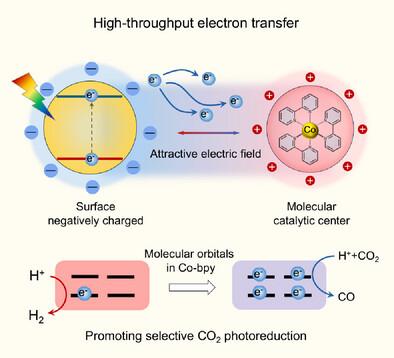
无机-有机界面电场中的高通量电子转移使CO2选择性光还原
长期以来,光催化CO2还原的效率一直受到H2演化反应的限制。在这项研究中,我们提出了一种创新的策略来促进高通量电子转移来抑制H2的演化,从而增强CO2的还原。通过使用cd和联吡啶钴作为模型,我们设计了cd的表面,在无机-有机界面处产生电场。通过原位和瞬态光谱技术,我们发现,与那些与─NH2基团功能化的CdS相比,与COOH基团功能化的CdS表现出显著的非共价相互作用和改进的电荷转移能力。电子在联吡啶钴上的快速传递有利于吸附的CO2参与质子-电子耦合反应,而不是让被吸附的质子直接接受电子。结果表明,所建立的CdS-COOH/Co(II)-bpy体系Co产率为2.523 mmol g−1 h−1,选择性为96.3%。本研究提出了一种创建高效电荷传输界面的方法,并为设计高性能光催化CO2还原系统提供了一种全面的策略,该系统可以有效地抵消竞争性H2演化反应带来的挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


