Biotin-guided NIR fluorescent probe for in situ imaging of fibroblast activation protein in human breast cancer tissues
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
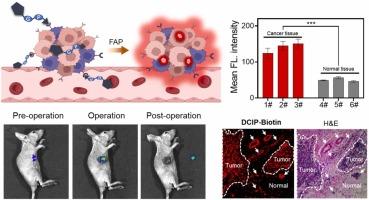
Breast cancer continues to be the malignant tumor with the highest incidence and mortality rates among women. Fibroblast activation protein (FAP) is a leading diagnostic and therapeutic biomarker associated with immune suppression, cancer metastasis, and poor prognosis in solid cancers, particularly in breast cancer. Therefore, developing a FAP-activated fluorescent probe holds considerable promise for the early detection and therapy of breast cancer. In this work, we designed a tumor-targeted near-infrared (NIR) fluorescent probe, DCIP-Biotin, to detect FAP activity in human breast cancer cells, tumor-bearing mice and human breast cancer tissue samples. The probe incorporated a novel NIR fluorophore (dicyanoisophorone) with an FAP recognition substrate linked to a biotin tag. The probe exhibited a wider linear range, high sensitivity, good selectivity, and robust anti-interference capability. DCIP-Biotin could be specifically activated at 660 nm when FAP was present, enabling it to efficiently recognize and image FAP-expressing cells. Furthermore, excellent fluorescence imaging performance of DCIP-Biotin was observed in MDA-MB-231 tumor-bearing mice and human breast cancer tissues, including high specificity, a high tumor-to-background ratio, deep tissue penetration, and stable fluorescence signal. DCIP-Biotin exhibits such good performance that it allows for clear demarcation between human breast cancer and adjacent non-cancerous regions, demonstrating considerable promise for use in intraoperative navigation.
生物素引导的近红外荧光探针用于人乳腺癌组织成纤维细胞活化蛋白的原位成像
乳腺癌仍然是妇女发病率和死亡率最高的恶性肿瘤。成纤维细胞激活蛋白(FAP)是一种领先的诊断和治疗生物标志物,与实体癌(尤其是乳腺癌)的免疫抑制、癌症转移和不良预后相关。因此,开发一种fap激活的荧光探针对乳腺癌的早期发现和治疗具有相当大的希望。本研究设计了一种肿瘤靶向近红外(NIR)荧光探针DCIP-Biotin,用于检测人乳腺癌细胞、荷瘤小鼠和人乳腺癌组织样本中的FAP活性。该探针结合了一种新型的近红外荧光团(二氰异佛尔酮),其FAP识别底物与生物素标签相连。该探针具有线性范围宽、灵敏度高、选择性好、抗干扰能力强等特点。当FAP存在时,DCIP-Biotin在660 nm处被特异性激活,能够有效识别和成像表达FAP的细胞。此外,DCIP-Biotin在MDA-MB-231荷瘤小鼠和人乳腺癌组织中表现出良好的荧光成像性能,具有高特异性、高瘤本底比、组织穿透深、荧光信号稳定等特点。DCIP-Biotin表现出如此良好的性能,它可以明确区分人类乳腺癌和邻近的非癌区域,在术中导航中显示出相当大的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


