Hydrogen storage via clathrate hydrates under mild conditions enabled by mixed cyclic ether synergy
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Hydrogen (H2) storage via clathrate hydrates represents a compelling strategy for sustainable energy solutions. However, slow formation kinetics and rigorous thermodynamic conditions pose significant barriers to their widespread implementation. Here, we present a molecularly tailored approach using mixed cyclic ether promoters, 1,3-dioxane (DXN) and 1,3-dioxolane (DIOX), in deuterium oxide (D2O) to significantly enhance H2 storage under mild, and practically relevant conditions. We tasted H2 enclathration across various parameters, including promoter ratios, formation temperatures, promoter concentrations, multiple reformation cycles, and initial H2 pressures. Our optimized formulation 2DXN-1DIOX (5.56 mol%) achieves superior H2 uptake of 43.77 43.77 ± 2.68 mmol H2/mol D2O at 274.45 K and 12.5 MPa, which reproducibly increases to 56.12 ± 0.58 mmol H2/mol D2O at 18.5 MPa, driven by structural and kinetic synergy between DXN and DIOX molecules. Powder X-ray diffraction confirmed the formation of structure II (sII) hydrates, while Raman spectroscopy elucidated molecular-level details of structural heterogeneity, revealing how promoter-induced lattice flexibility enhances H2 diffusion and promotes efficient hydrate cage accessibility within the hydrate framework. Complementary thermodynamic phase equilibrium investigations established that the mixed-promoter system considerably expands the hydrate stability envelope toward milder pressures and temperatures, further highlighting its potential. Collectively, this work reveals how mixed cyclic ether promoters synergistically enhance H2 enclathration and hydrate stability under practical conditions, thereby providing a molecular design framework to advance clathrate-based H2 storage toward commercial viability.
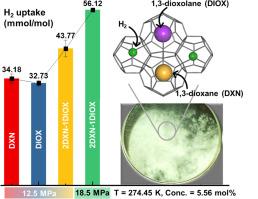
混合循环醚协同作用在温和条件下通过笼形水合物储氢
通过笼形水合物储存氢(H2)代表了可持续能源解决方案的一个引人注目的战略。然而,缓慢的地层动力学和严格的热力学条件对其广泛应用构成了重大障碍。在这里,我们提出了一种分子定制的方法,在氧化氘(D2O)中使用混合环醚促进剂1,3-二氧六环烷(DXN)和1,3-二氧六环烷(DIOX),在温和且实际相关的条件下显著提高H2储存。我们在不同的参数下,包括启动子比、形成温度、启动子浓度、多次重组周期和初始H2压力,对H2的笼化进行了实验。我们优化配方2 dxn-1diox(5.56 摩尔%)达到优越的H2吸收43.77 - 43.77 ±2.68 更易与H2 /摩尔D2O 274.45 K和12.5 MPa,这可再生产地增加到56.12 ±0.58 更易与H2 /摩尔D2O 18.5 MPa,由结构和动力学DXN和DIOX分子之间的协同作用。粉末x射线衍射证实了结构II (sII)水合物的形成,而拉曼光谱揭示了结构非均质性的分子水平细节,揭示了启动子诱导的晶格柔韧性如何增强H2扩散并促进水合物框架内水合物笼的有效接近。互补的热力学相平衡研究表明,混合促进剂体系在较温和的压力和温度下显著扩展了水合物的稳定性包络,进一步凸显了其潜力。总的来说,这项工作揭示了混合环醚促进剂如何在实际条件下协同增强H2包合和水合物稳定性,从而提供了一个分子设计框架,以推进基于包合物的H2储存走向商业可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


