Endogenous stimuli-derived self-assembled DNA tetrahedron (MESH) for precise imaging and activatable mitochondrial interference therapy
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Cancer remains a great danger for health and well-being as well as a challenge for the sustainability of Health Systems worldwide. At the same time, tumor theranostics are hampered by limitations in imaging sensitivity, inadequate specificity, side effects and the emergence of therapeutic resistance. Tumor endogenous-activatable theranostic probes have emerged as critical tools for advancing precision diagnostics and targeted treatment of aggressive malignancies. In this study, we developed an endogenous stimuli-derived self-assembled DNA tetrahedron (MESH) nanodevice for simultaneous tumor visualization and activatable mitochondrial interference therapy. The DNA tetrahedron precisely recognized cancer cells via the Mucin-1 (MUC1) aptamer, and tumor-derived microRNA activated a strand displacement cascade amplification reaction to enable specifically and sensitively fluorescence imaging of malignant lesions. Concurrently, the in situ self-assembly process of DNA tetrahedron was initiated to form a DNA network under the stimulation of microRNA in cytoplasm. The self-assembled DNA network could selectively localize to mitochondria, acting as a polyanionic barrier that disrupts mitochondrial function and induces apoptosis. This endogenous tumor microenvironment-regulated morphological transformation between biocompatible DNA tetrahedral and DNA network with suborganelle interference functions might address the side effects and resistance issues of tumor treatment. The MESH provided a novel strategy for cancer imaging and mitochondrial manipulation through endogenous molecular-guided assembly with potential applications in theranostics.
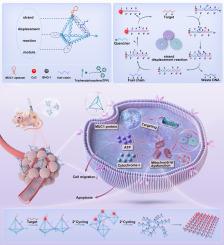
内源性刺激衍生的自组装DNA四面体(MESH)用于精确成像和可激活线粒体干扰治疗
癌症仍然是对健康和福祉的巨大威胁,也是对全球卫生系统可持续性的挑战。与此同时,肿瘤的治疗受到成像敏感性的限制、特异性不足、副作用和治疗耐药性的出现的阻碍。肿瘤内源激活治疗探针已成为推进精确诊断和靶向治疗侵袭性恶性肿瘤的关键工具。在这项研究中,我们开发了一种内源性刺激衍生的自组装DNA四面体(MESH)纳米装置,用于同时观察肿瘤和激活线粒体干扰治疗。DNA四面体通过Mucin-1 (MUC1)适体精确识别癌细胞,肿瘤来源的microRNA激活链位移级联扩增反应,从而特异性和敏感地对恶性病变进行荧光成像。同时,在细胞质中microRNA的刺激下,启动DNA四面体的原位自组装过程,形成DNA网络。自组装的DNA网络可以选择性地定位到线粒体,作为一个多阴离子屏障,破坏线粒体功能并诱导细胞凋亡。这种内源性肿瘤微环境调控的生物相容性DNA四面体与具有亚细胞器干扰功能的DNA网络之间的形态转化可能解决肿瘤治疗的副作用和耐药问题。MESH通过内源性分子引导组装为癌症成像和线粒体操作提供了一种新的策略,在治疗学中具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


