Pyrogenic carbon-stimulated nitrate-dependent anaerobic methane oxidation: insights into redox activity and conductivity in anaerobic methanotrophic archaea metabolism and microbial dynamics
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Pyrogenic carbon (PC) plays a critical role in regulating greenhouse gas emissions by influencing methanogenesis and methane oxidation in aquatic environments. However, its impact on nitrate-dependent anaerobic oxidation of methane (AOM), associated methane emissions, and the underlying mechanisms remain poorly understood. Here, we demonstrated that in nitrate-dependent AOM consortia amended with HNO3-treated biochar and graphite (representing redox-active and conductive forms of PC, respectively), AOM rates were significantly elevated by 2.7- and 4.4-fold, respectively, compared to unamended biotic controls. This enhancement was accompanied by a pronounced proliferation of anaerobic methanotrophic archaea, specifically “Candidatus Methanoperedens nitroreducens”, along with elevated metabolic activity driven by enhanced electron transport and energy conservation, as indicated by significantly increased electron transfer system activity, total adenine nucleotide levels, and concentrations of key redox carrier F420. Metagenomic analysis revealed that PC addition reshaped microbial interactions. Notably, graphite facilitated the potential establishment of direct interspecies electron transfer between “Ca. M. nitroreducens” and coexisting denitrifying populations (Bacteroidota sp. and Ignavibacteriaceae sp.), while also fostering the formation of new interspecies networks that enabled division of labor within the denitrification pathway. These findings not only advance the mechanistic understanding of PC-facilitated methane mitigation in aquatic ecosystems but also suggest strategies for engineering AOM-based systems to optimize methane removal and nitrogen cycling in environmental applications.
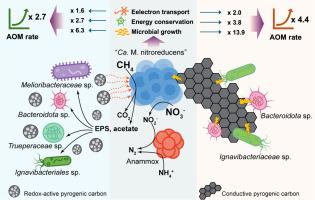
热原碳刺激硝酸盐依赖厌氧甲烷氧化:对厌氧甲烷营养古菌代谢和微生物动力学中的氧化还原活性和电导率的见解
热原碳(PC)通过影响水生环境的甲烷生成和甲烷氧化,在调节温室气体排放中起着至关重要的作用。然而,其对硝酸盐依赖性甲烷厌氧氧化(AOM)的影响、相关的甲烷排放以及潜在的机制仍然知之甚少。在这里,我们证明了用hno3处理过的生物炭和石墨(分别代表氧化还原活性和导电形式的PC)修饰的硝酸盐依赖性AOM群落中,与未修饰的生物对照相比,AOM率分别显著提高了2.7倍和4.4倍。这种增强伴随着厌氧甲烷营养古菌(特别是“Candidatus Methanoperedens nitroreducens”)的显著增殖,以及电子传递和能量节约增强驱动的代谢活性升高,如电子传递系统活性、总腺嘌呤核苷酸水平和关键氧化还原载体F420浓度的显著增加。宏基因组分析显示,添加PC重塑了微生物相互作用。值得注意的是,石墨促进了“Ca. M. nitroreducens”和共存的反硝化种群(Bacteroidota sp.和Ignavibacteriaceae sp.)之间直接种间电子转移的潜在建立,同时也促进了新的种间网络的形成,使反硝化途径内的劳动分工成为可能。这些发现不仅促进了对pc促进的水生生态系统甲烷缓解机制的理解,而且为基于aom的工程系统提供了策略,以优化环境应用中的甲烷去除和氮循环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


