Fe2N-modulated Mo2N/MoO2 multiple heterostructures for optimized electron configuration and reaction dynamics in alkaline hydrogen evolution reaction
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Mo-based catalysts have received widespread attention for their promising hydrogen evolution reaction (HER) efficiency. However, single Mo-based catalysts still have some shortcomings in terms of catalytic activity. Designing heterostructure is an effective strategy to optimize electron configuration, accelerate HER dynamics and modulate microstructure. Herein, a novel cross-linked sheet-like r-FeMo/NF (Fe2N/Mo2N/MoO2) electrocatalyst with multiple heterostructures was prepared by a facile two-step method involving hydrothermal growth followed by nitridation, which directly formed the active material on nickel foam (NF). The formation of multiple heterostructures results in the redistribution of electrons between Fe and Mo species, promoting charge transfer and improving HER activity. As a result, the as-prepared r-FeMo/NF catalyst demands a low overpotential of 46.1 mV to drive the current density of 10 mA cm−2 for the HER in 1 M KOH. Moreover, it demonstrates outstanding long-term stability, maintaining excellent performance for 100 h at both 10 and 100 mA cm−2. This work can provide valuable insights into designing highly efficient electrocatalysts with multiple heterostructures.
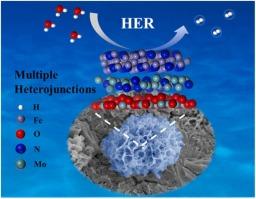
fe2n调制Mo2N/MoO2多异质结构优化电子组态及碱性析氢反应动力学
钼基催化剂因其具有良好的析氢反应效率而受到广泛关注。然而,单钼基催化剂在催化活性方面还存在一些不足。异质结构设计是优化电子组态、加速HER动力学和调节微结构的有效策略。本文采用水热生长-氮化两步法制备了一种新型的具有多异质结构的交联片状r-FeMo/NF (Fe2N/Mo2N/MoO2)电催化剂,该催化剂直接在泡沫镍(NF)上形成活性物质。多种异质结构的形成导致电子在Fe和Mo之间重新分布,促进电荷转移,提高HER活性。结果表明,制备的r-FeMo/NF催化剂需要低过电位46.1 mV,才能在1 M KOH条件下驱动HER的电流密度为10 mA cm-2。此外,它还具有出色的长期稳定性,在10和100 mA cm-2下均能保持100小时的优异性能。这项工作为设计高效的多异质结构电催化剂提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


