Comparative study on hydration shell structure and wettability characteristics of Quartz, Feldspar, and muscovite surfaces
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Solid–liquid interfaces of silicate minerals, particularly mineral–water interfacial interactions, have been found to directly influence the processes of mineral material modification, ion transport, and flotation separation. We systematically investigated the effects of surfactants on the hydration shells of quartz, feldspar, and muscovite based on large-scale molecular dynamic simulations, flotation kinetics, and contact angle. Muscovite exhibited a comparatively thicker hydration shell with looser water molecule arrangement and greater hydrophilicity than those of quartz and feldspar, which may lead to a much lower contact angle of 9.48°. The contact angles of the three minerals increased rapidly with the addition of dodecylamine (DDA). However, the adsorption of DDA on the silicate minerals formed holes through its hydrophobic alkyl chains, indicating that the hydration shells would return to their original states after removal of DDA. Feldspar and muscovite had similar flotation constants, whereas those of muscovite and quartz were distinct, which may be attributed to the difference in the adsorption sites of DDA on the minerals and the density and orientation of water molecules in their hydration shells. This study provides a comparative analysis of the hydration shell features between the silicate minerals, establishing a new perspective for understanding the interfacial phenomena in a mineral–aqueous solution.
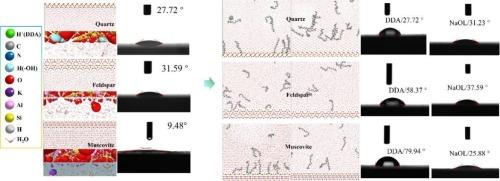
石英、长石和白云母表面水化壳结构及润湿性的比较研究
硅酸盐矿物的固液界面,特别是矿物-水界面的相互作用,直接影响矿物材料改性、离子传输和浮选分离的过程。基于大规模分子动力学模拟、浮选动力学和接触角,我们系统地研究了表面活性剂对石英、长石和白云母水化壳的影响。与石英和长石相比,白云母的水化壳相对较厚,水分子排列更松散,亲水性更强,这可能导致接触角远低于石英和长石的9.48°。随着十二烷基胺(DDA)的加入,三种矿物的接触角迅速增大。然而,DDA在硅酸盐矿物上的吸附通过其疏水性烷基链形成孔洞,表明去除DDA后水化壳会恢复到原来的状态。长石和白云母的浮选常数相似,而白云母和石英的浮选常数不同,这可能与DDA在矿物上的吸附位置以及水化壳中水分子的密度和取向不同有关。本研究对硅酸盐矿物水化壳特征进行了对比分析,为理解矿物-水溶液中的界面现象建立了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


