Effect of temperature on the aggregation of an Fc-fusion protein under agitation
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Mitigating protein aggregation remains a challenge in the development of biopharmaceuticals, and agitation is well known as a stress that can induce protein aggregation. However, the temperature dependence of agitation-induced aggregation is not well understood. In this study, the aggregation of an Fc-fusion protein under agitation stress was investigated at 5, 25, and 40 °C. Soluble and insoluble aggregates were quantified by size-exclusion liquid chromatography and flow imaging microscopy, respectively. Both the aggregation level and the aggregate clusters were temperature dependent. The threshold for the orbital shaking that induced protein aggregation was temperature independent. Although thermal stress at 40 °C increased the number of oligomers, it did not lead to a higher monomer loss in a subsequent agitation at 25 °C. The aggregation induced by agitation stress was suppressed by adding a surfactant or removing the vial headspace, indicating that the aggregation occurred via an interface-mediated pathway. Thus, the observed temperature dependence was attributed to the protein adsorption to the interface and the following interfacial unfolding and aggregation was affected by the temperature. The results emphasized the importance of temperature control during shipping to ensure the quality of drug products. Agitation stability studies at a controlled temperature also provide a deep understanding of the protein aggregation mechanism, which is important for formulation development.
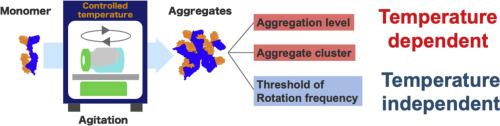
温度对搅拌下fc融合蛋白聚集的影响。
减轻蛋白质聚集仍然是生物制药发展中的一个挑战,搅拌是众所周知的一种可以诱导蛋白质聚集的压力。然而,温度依赖性的搅拌诱导聚集还不是很清楚。在这项研究中,研究了在5、25和40°C搅拌胁迫下fc融合蛋白的聚集。溶性和不溶性聚集体分别用排粒径液相色谱法和流动成像显微镜进行定量。聚集水平和聚集簇都与温度有关。引起蛋白质聚集的轨道震动阈值与温度无关。虽然40°C的热应力增加了低聚物的数量,但在随后的25°C搅拌中,它并没有导致更高的单体损失。通过添加表面活性剂或去除瓶顶空间可以抑制搅拌应力引起的聚集,表明聚集是通过界面介导的途径发生的。因此,观察到的温度依赖性归因于蛋白质在界面上的吸附,随后的界面展开和聚集受温度的影响。结果强调了运输过程中温度控制对保证药品质量的重要性。在受控温度下的搅拌稳定性研究也提供了对蛋白质聚集机制的深入了解,这对配方开发至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


