A new UDP-glycosyltransferase for rare ginsenoside biosynthesis from Gynostemma pentaphyllum (Thunb.)
IF 2.5
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Heterologous biosynthesis of ginsenosides, which possess remarkable therapeutic potential as drug candidates, is currently a research hotspot. The insufficient mining of UDP-glycosyltransferases (UGTs), which are key downstream enzymes in their biosynthetic pathway, limits the variety and yield of ginsenosides that can be bio-produced. As the only medicinal plant outside the Araliaceae family currently discovered to contain ginsenosides, the enzymes involved in ginsenoside synthesis in the Cucurbitaceae plant Gynostemma pentaphyllum (Thunb.) have great development value. In this study, a new glycosyltransferase was mined from G. pentaphyllum and was classified into the UGT74 family based on sequence homology, named GpUGT74A1. GpUGT74A1 exhibits relatively low sequence homology compared to other UGTs that have been reported. Although GpUGT74A1 was almost entirely insoluble when cloned and expressed in Escherichia coli, its soluble expression was successfully achieved by fusion with the MBP solubility tag. In vitro enzyme activity experiments found that it could catalyze the C-20 glycosylation of ginsenosides PPD, PPT, and Rh2 to produce ginsenosides CK, F1, and F2, respectively. This study further enriched the sequence of plant-derived glycosyltransferase genes and provided new candidate elements for the heterologous synthesis of rare ginsenosides.
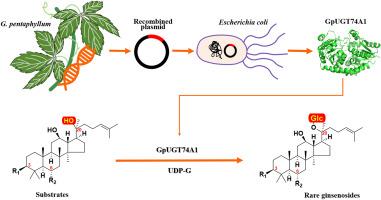
一种新的用于绞股蓝合成稀有人参皂苷的udp -糖基转移酶。
人参皂苷的异源生物合成作为候选药物具有显著的治疗潜力,是目前的研究热点。作为生物合成途径中关键的下游酶,udp -糖基转移酶(UGTs)的开采不足,限制了人参皂苷生物生产的品种和产量。绞股蓝(Gynostemma pentaphyllum, Thunb.)是目前发现的除五加科外唯一含有人参皂苷的药用植物,其合成人参皂苷的酶具有很大的开发价值。本研究从G. pentaphyllum中分离到一种新的糖基转移酶,根据序列同源性将其归入UGT74家族,命名为GpUGT74A1。与其他已报道的ugt相比,GpUGT74A1的序列同源性相对较低。虽然GpUGT74A1在大肠杆菌中克隆表达时几乎完全不溶,但通过与MBP溶解度标签融合,成功实现了其可溶性表达。体外酶活性实验发现,它可以催化人参皂苷PPD、PPT和Rh2的C-20糖基化,分别生成人参皂苷CK、F1和F2。该研究进一步丰富了植物源性糖基转移酶基因序列,为稀有人参皂苷的异源合成提供了新的候选元件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


