Study on the carbothermal reduction process of SiO2-Fe2O3-Al2O3-CaO system in coal gasification fine slag
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-05-10
DOI:10.1016/j.jiec.2025.04.057
引用次数: 0
Abstract
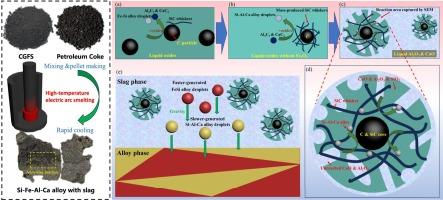
The Si-Fe-Al-Ca alloy preparation from coal gasification fine slag (CGFS) by high-temperature carbothermal reduction provides an effective way to realize the high-value utilization of solid waste, solve environmental problems caused by waste accumulation, and reduce the production cost of ferrosilicon alloys. However, the carbothermal reduction mechanism of the complex quaternary SiO2-Fe2O3-Al2O3-CaO system in CGFS is still unclear, which is difficult to explain by existing theories. In this study, CGFS was used with petroleum coke as an additional reducing agent to prepare Si-Fe-Al-Ca alloys by high-temperature carbothermal reduction, and the intermediate state of the smelting process was observed by slice analysis of the rapidly cooled product. The carbothermal reduction process and mechanism of the SiO2-Fe2O3-Al2O3-CaO quaternary oxide system were discussed in depth in conjunction with thermodynamic calculations by FactSage software. The results show that the carbothermal reduction process of the SiO2-Fe2O3-Al2O3-CaO system can be divided into three stages, the first stage of rapid generation FeSi and SiC; the second stage of the reaction of a small amount of Al4C3 and CaC2 with SiO2; the third stage of the reaction of CaO, Al2O3 with SiC. In the third stage, the reaction of SiC with CaO and Al2O3 is the main reaction to generate Ca and Al. The mass-generated SiC whiskers block the C particles from oxides causing the difficulty of smelting CGFS. The complexity of the phase composition of the alloy is due to the generation of Fe-Si alloy and Si-Al-Ca alloy at different stages of the reaction, which alternately fell into the alloy melt pool with gravity.
煤气化细渣中SiO2-Fe2O3-Al2O3-CaO体系碳热还原过程研究
煤气化细渣高温碳热还原法制备Si-Fe-Al-Ca合金,为实现固体废弃物高价值利用、解决废弃物堆积带来的环境问题、降低硅铁合金生产成本提供了有效途径。然而,复合季元SiO2-Fe2O3-Al2O3-CaO体系在CGFS中的碳热还原机理尚不清楚,现有理论难以解释。本研究将CGFS与石油焦作为附加还原剂,采用高温碳热还原法制备Si-Fe-Al-Ca合金,并通过快速冷却产物的切片分析观察熔炼过程的中间状态。结合FactSage软件的热力学计算,对SiO2-Fe2O3-Al2O3-CaO季氧化体系的碳热还原过程和机理进行了深入探讨。结果表明:SiO2-Fe2O3-Al2O3-CaO体系的碳热还原过程可分为3个阶段,第一阶段快速生成FeSi和SiC;第二阶段是少量Al4C3和CaC2与SiO2的反应;CaO、Al2O3与SiC反应的第三阶段。在第三阶段,SiC与CaO和Al2O3的反应是生成Ca和Al的主要反应。大量生成的SiC晶须阻挡了C颗粒与氧化物的接触,导致CGFS冶炼困难。合金相组成的复杂性是由于在反应的不同阶段生成了Fe-Si合金和Si-Al-Ca合金,它们在重力作用下交替落入合金熔池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


