Shortening sulfur redox pathway via coupled LiO and CS bonds in LiS batteries
IF 8.9
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
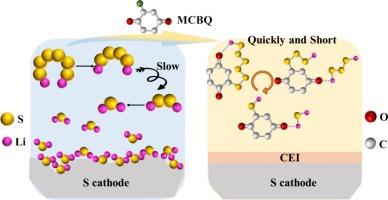
The “shuttle effect,” slow sulfur redox kinetics, and low utilization of active materials have become challenges that lithium‑sulfur (Li![]() S) batteries must currently address. To tackle these issues, this study employs 2-chloro-1,4-benzoquinone (MCBQ) as an electrolyte additive. MCBQ, with its carbonyl oxygen structure and halogen substituents, can react with polysulfides to form a cyclic insoluble organic sulfur intermediate that contains Li
S) batteries must currently address. To tackle these issues, this study employs 2-chloro-1,4-benzoquinone (MCBQ) as an electrolyte additive. MCBQ, with its carbonyl oxygen structure and halogen substituents, can react with polysulfides to form a cyclic insoluble organic sulfur intermediate that contains Li![]() O bonds and C
O bonds and C![]() S bonds. During discharge, it alters the conversion pathway of sulfur species. Taking Li2S6 as an example, the original path “Li2S6 → Li2S4 → Li2S2 → Li2S” is optimized into a new pathway “MCBQ- Li2S6 → MCBQ-2Li2S3 → MCBQ-2Li2S” simplifying the route to enhance sulfur redox kinetic efficiency. Moreover, MCBQ also forms an organic-inorganic hybrid interfacial protective layer on the lithium anode side, effectively mitigating the corrosion of the lithium anode by polysulfides. The battery with the MCBQ additive maintains a Coulombic efficiency of 100 % at a current density of 0.2C, with an initial discharge capacity reaching 812 mAh g−1; even after 120 cycles, the capacity decay rate per cycle is only 0.42 %. The introduction of MCBQ not only accelerates sulfur redox kinetics but also enhances the utilization of active materials and overall battery stability.
S bonds. During discharge, it alters the conversion pathway of sulfur species. Taking Li2S6 as an example, the original path “Li2S6 → Li2S4 → Li2S2 → Li2S” is optimized into a new pathway “MCBQ- Li2S6 → MCBQ-2Li2S3 → MCBQ-2Li2S” simplifying the route to enhance sulfur redox kinetic efficiency. Moreover, MCBQ also forms an organic-inorganic hybrid interfacial protective layer on the lithium anode side, effectively mitigating the corrosion of the lithium anode by polysulfides. The battery with the MCBQ additive maintains a Coulombic efficiency of 100 % at a current density of 0.2C, with an initial discharge capacity reaching 812 mAh g−1; even after 120 cycles, the capacity decay rate per cycle is only 0.42 %. The introduction of MCBQ not only accelerates sulfur redox kinetics but also enhances the utilization of active materials and overall battery stability.
通过锂离子电池中LiO和CS键的耦合缩短硫氧化还原途径
“穿梭效应”、硫氧化还原动力学缓慢以及活性材料利用率低已成为锂硫电池目前必须解决的挑战。为了解决这些问题,本研究采用2-氯-1,4-苯醌(MCBQ)作为电解质添加剂。MCBQ具有羰基氧结构和卤素取代基,可与多硫化物反应形成含LiO键和CS键的环不溶性有机硫中间体。在放电过程中,它改变了硫的转化途径。以Li2S6为例,将原路径“Li2S6→Li2S4→Li2S2→Li2S”优化为“MCBQ- Li2S6→MCBQ- 2li2s3→MCBQ- 2li2s”,简化了路径,提高了硫的氧化还原动力学效率。此外,MCBQ还在锂阳极侧形成有机-无机杂化界面保护层,有效减轻了多硫化物对锂阳极的腐蚀。添加MCBQ的电池在电流密度为0.2C时,库仑效率保持在100%,初始放电容量达到812 mAh g−1;即使经过120次循环,每个循环的容量衰减率也只有0.42%。MCBQ的引入不仅加速了硫氧化还原动力学,而且提高了活性物质的利用率和电池的整体稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of energy storage
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
11.80
自引率
24.50%
发文量
2262
审稿时长
69 days
期刊介绍:
Journal of energy storage focusses on all aspects of energy storage, in particular systems integration, electric grid integration, modelling and analysis, novel energy storage technologies, sizing and management strategies, business models for operation of storage systems and energy storage developments worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


