Non-lethal sonodynamic therapy-engineered foam cell exosomes reprograms endothelial immunometabolic crosstalk to stabilize atherosclerotic plaques
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Objective
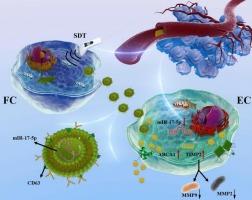
While non-lethal sonodynamic therapy (NL-SDT) demonstrates therapeutic potential for atherosclerosis, its immunomodulatory mechanisms via exosomal signaling remain unexplored. This study investigates how SDT-engineered foam cell exosomes orchestrate plaque stabilization through coordinated immunometabolic reprogramming of endothelial inflammation and matrix dynamics.
Methods
Atherosclerotic apolipoprotein E-deficient mice received NL-SDT with GW4869-mediated exosome blockade to confirm SDT-exosome axis dependency. Exosomes from bone marrow-derived foam cells were isolated, sequenced, and functionally validated via miR-17-5p gain/loss-of-function experiments. Dual-luciferase reporter assays confirmed miR-17-5p targeting of ABCA1 (cholesterol metabolism) and TIMP2 (inflammatory matrix regulation). Endothelial cholesterol efflux (using BODIPY-cholesterol) and MMP2/MMP9 levels were quantified.
Results
SDT-engineered foam cell exosomes showed endothelial tropism, delivering anti-inflammatory miR-17-5p suppression. Through miR-17-5p downregulation, SDT-engineered exosomes activated ABCA1-dependent cholesterol efflux and enhanced TIMP2-mediated MMP inhibition.
Conclusion
We identify SDT-engineered exosomes as novel immunotherapeutic vectors that synchronize metabolic detoxification and immune resolution in atherosclerotic plaques. Mechanistically distinct from direct exosome therapies, SDT amplifies endogenous exosome production while engineering cargo specificity, offering a tunable, lesion-targeted strategy.
非致死性声动力治疗工程泡沫细胞外泌体重编程内皮免疫代谢串扰以稳定动脉粥样硬化斑块
虽然非致死性声动力疗法(NL-SDT)显示出治疗动脉粥样硬化的潜力,但其通过外泌体信号传导的免疫调节机制仍未被探索。本研究探讨了sdt工程泡沫细胞外泌体如何通过内皮炎症和基质动力学的协调免疫代谢重编程来协调斑块稳定。方法用gw4869介导的外泌体阻断法对动脉硬化性载脂蛋白e缺陷小鼠进行NL-SDT治疗,以证实sdt -外泌体轴依赖性。从骨髓来源的泡沫细胞中分离外泌体,测序,并通过miR-17-5p功能增益/丧失实验进行功能验证。双荧光素酶报告基因检测证实了miR-17-5p靶向ABCA1(胆固醇代谢)和TIMP2(炎症基质调节)。内皮胆固醇外排(使用bodipy -胆固醇)和MMP2/MMP9水平被量化。结果ssdt工程泡沫细胞外泌体显示内皮性,传递抗炎miR-17-5p抑制。通过下调miR-17-5p, sdt工程外泌体激活了abca1依赖性胆固醇外排,增强了timp2介导的MMP抑制。结论:我们发现sdt工程外泌体是一种新的免疫治疗载体,可以同步动脉粥样硬化斑块的代谢解毒和免疫溶解。从机制上不同于直接的外泌体治疗,SDT放大了内源性外泌体的产生,同时工程货物特异性,提供了一种可调的病变靶向策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


