Optimization of adsorption parameters of bromocresol green dye and Acacia mangium wood activated carbon: Kinetics, thermodynamics, isotherm, and surface interaction mechanism
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study aimed to optimize the adsorption capacity of Acacia mangium wood activated carbon (AMW-AC) against bromocresol green (BCG) dye using a face-centered central composite design of response surface methodology (RSM). The variables considered for optimization are AMW-AC dosage (0.5-1.5 g/L), contact time (30-120 min), and BCG dye initial concentration (50-300 mg/L) to maximize the adsorption capacity (mg/g) of AMW-AC. The statistical design resulted in a quadratic model with only two significant factors: adsorbent dosage and initial concentration of BCG dye. The contact time was not an important factor because the adsorption was quick, and maximum adsorption occurred within 30 minutes of the contact time. The adsorption capacity data fit well in the proposed RSM model, which indicates that the control change in independent variables (AMW-AC dose, contact time, and initial BCG dye concentration) regulated the variability in the response (adsorption capacity). The optimal value of independent variables for the maximum adsorption capacity of AMW-AC (580.4 ± 11.4 mg/g) was 0.5 g/L of AMW-AC dosage, 30 min of contact time, and 300 mg/L of BCG dye concentration. The kinetic data of BCG dye adsorption followed the pseudo-second order model, and the isotherm data fit well with the Freundlich isotherm model (predicted qmax 586.22 mg/g). Thermodynamic evaluation of BCG dye adsorption data revealed that adsorption was exothermic and spontaneous. This study also illustrated the BCG adsorption mechanism. The AMW-AC adsorbent surface is mostly comprised of C, N, O, and P-based functional groups. The adsorption mechanism between the BCG molecules and the AMW-AC surface involved chemical functional group interactions and the entrapment of hydrated dye molecules in the pores. The energy values calculated from the isotherms and thermodynamics stated that the adsorption of BCG dye onto the AMW-AC was due to a weak van der Waals force.
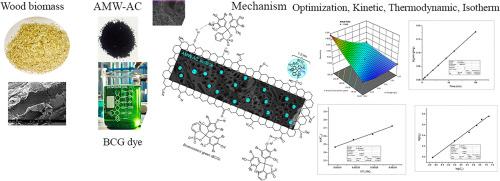
溴甲酚绿色染料与马相思木活性炭吸附参数的优化:动力学、热力学、等温线及表面相互作用机理
采用响应面法(RSM)的面心中心复合设计优化了相思木活性炭(AMW-AC)对溴甲酚绿(BCG)染料的吸附性能。以AMW-AC的用量(0.5 ~ 1.5 g/L)、接触时间(30 ~ 120 min)和BCG染料初始浓度(50 ~ 300 mg/L)为优化变量,使AMW-AC的吸附量(mg/g)达到最大。统计设计的结果是一个二次模型,只有两个显著因素:吸附剂用量和BCG染料的初始浓度。接触时间不是影响吸附的重要因素,因为吸附速度快,在接触时间后30分钟内吸附量最大。吸附容量数据与所提出的RSM模型拟合良好,表明自变量(AMW-AC剂量、接触时间和初始BCG染料浓度)的控制变化调节了响应(吸附容量)的变异性。AMW-AC最大吸附量(580.4±11.4 mg/g)的自变量最佳值为:AMW-AC用量0.5 g/L、接触时间30 min、BCG染料浓度300 mg/L。吸附BCG染料的动力学数据符合拟二阶模型,等温线数据符合Freundlich等温线模型(预测qmax为586.22 mg/g)。对卡介苗染料吸附数据的热力学评价表明,吸附是自发的、放热的。本研究还阐明了BCG的吸附机理。AMW-AC吸附剂表面主要由C、N、O和p基官能团组成。BCG分子与AMW-AC表面的吸附机制包括化学官能团相互作用和水合染料分子在孔隙中的吸附。等温线和热力学计算的能量值表明,卡介苗染料在AMW-AC上的吸附是由于微弱的范德华力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


