Model-driven high-level expression of β-mannanase in Pichia pastoris for degrading mannan in palm kernel expeller
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-09-25
DOI:10.1016/j.carpta.2025.101017
引用次数: 0
Abstract
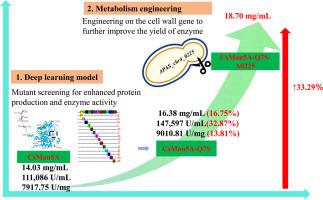
Palm kernel expeller (PKE), a copious byproduct, is limited in its application to non-ruminant diets due to the presence of antinutritive factors, predominantly galactomannan, necessitating effective enzymatic hydrolysis by β-mannanase to enhance its nutritional value. Here, a cross-strategy combining the predicting mutations with enhanced protein expression (PMEPE) model with B-factor analysis was employed to rationally modify the gene encoding β-mannanase CsMan5A in Pichia pastoris. The expression level of mutant CsMan5A-Q7S improved 17 % compared to the wild type, reaching 16.38 g/L, which resulted in an increased enzyme activity titer of 147,597 U/mL and specific enzyme activity of 9010.81 U/mg, respectively increasing by 32.87 % and 13.81 % compared to the wild-type. Then, the expression of CsMan5A-Q7S was further elevated to 18.70 g/L through cell wall engineering. Computational simulations explained the mechanism of the superior thermostability and activity observed in the CsMan5A-Q7S mutant, which presented a more stable overall structure than the wild type. The residue left after hydrolysis by CsMan5A-Q7S had lower fiber content, as well as higher protein content, thereby effectively enhancing its feed quality. This study achieved exceptional phenotypic enhancement of β-mannanase production in Pichia pastoris by an effective strategy through computational modeling, protein engineering, and cell wall modification strategies.
模型驱动的β-甘露聚糖酶在毕赤酵母中的高水平表达,用于降解棕榈仁机中的甘露聚糖
棕榈仁提取物(PKE)是一种丰富的副产品,由于存在抗营养因子,主要是半乳甘露聚糖,因此在非反刍动物日粮中的应用受到限制,需要通过β-甘露聚糖酶有效地酶解以提高其营养价值。本研究采用预测突变与增强蛋白表达(PMEPE)模型结合b因子分析的交叉策略,对毕赤酵母β-甘露聚糖酶CsMan5A编码基因进行合理修饰。突变体CsMan5A-Q7S的表达量比野生型提高了17%,达到16.38 g/L,酶活性滴度为147,597 U/mL,比酶活性为9010.81 U/mg,分别比野生型提高了32.87%和13.81%。然后通过细胞壁工程将CsMan5A-Q7S的表达量进一步提高到18.70 g/L。计算模拟解释了在CsMan5A-Q7S突变体中观察到的优越的热稳定性和活性的机制,该突变体比野生型具有更稳定的整体结构。经CsMan5A-Q7S水解后的残渣纤维含量较低,蛋白质含量较高,有效提高了饲料品质。本研究通过计算建模、蛋白质工程和细胞壁修饰等有效策略,实现了毕赤酵母β-甘露聚糖酶生产的卓越表型增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


