Boosting ammonium-ion diffusion and cycling stability in PBAs via hydrogen bonding with interstitial water
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
Prussian blue analogs (PBAs) have emerged as environmentally friendly and structurally tunable cathode materials for aqueous ammonium-ion batteries (AIBs). However, the fundamental role of crystalline H2O in regulating ammonium-ion storage and transport remains poorly understood. In this study, we present a comprehensive comparison between hydrated NH4NiHCF-H2O and its anhydrous counterpart NH4NiHCF, revealing the critical contribution of interstitial water to electrochemical performance. Structural and spectroscopic analyses confirm that interstitial water forms robust hydrogen bonds with NH4+ ions, stabilizing the PBA framework and mitigating structural degradation during cycling. Electrochemical measurements show that NH4NiHCF-H2O delivers a significantly higher specific capacity of 61 mA h g−1 at 0.2 C and markedly improved rate performance compared to NH4NiHCF (48 mA h g−1 at 0.2 C). Kinetic analysis reveals that interstitial water enhances NH4+ diffusion, as evidenced by higher diffusion coefficients. Furthermore, density functional theory (DFT) calculations demonstrate that crystal water acts as a hydrogen bond acceptor, preferentially interacting with NH4+ and reducing the migration energy barrier, thereby facilitating fast ion transport. This work provides fundamental insights into the role of crystal water in PBAs and offers a rational design strategy for improving the kinetics, structural stability of PBAs cathodes for AIBs.
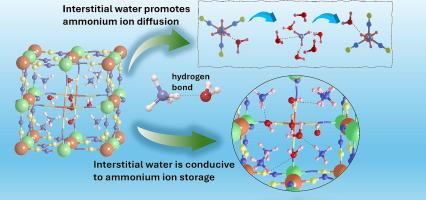
通过与间隙水的氢键增强氨离子在多环芳烃中的扩散和循环稳定性
普鲁士蓝类似物(PBAs)作为一种环境友好、结构可调的水铵离子电池(aib)正极材料已经出现。然而,晶体水在调节氨离子储存和运输中的基本作用仍然知之甚少。在这项研究中,我们对水合NH4NiHCF- h2o和无水NH4NiHCF进行了全面的比较,揭示了间隙水对电化学性能的重要贡献。结构和光谱分析证实,间隙水与NH4+离子形成坚固的氢键,稳定了PBA框架,减轻了循环过程中的结构降解。电化学测量表明,NH4NiHCF- h2o在0.2℃时的比容量为61 mA h g−1,比NH4NiHCF(0.2℃时为48 mA h g−1)有显著提高。动力学分析表明,间隙水增强了NH4+的扩散,扩散系数较高。此外,密度泛函理论(DFT)计算表明,结晶水作为氢键受体,优先与NH4+相互作用,降低迁移能垒,从而促进离子的快速传输。这项工作为结晶水在PBAs中的作用提供了基本的见解,并为改善AIBs PBAs阴极的动力学和结构稳定性提供了合理的设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


