Local charge redistribution-induced OER mechanism switching in RuO2-based catalysts for efficient PEM electrolysis
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
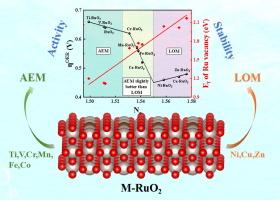
Oxygen evolution reaction (OER) is widely recognized as a bottleneck of water electrolysis. To determine the underlying reaction mechanisms, particularly the relative contribution of the adsorbate evolution mechanism (AEM) and lattice-oxygen participation mechanism (LOM), we conduct a comprehensive investigation combining Density Functional Theory (DFT) calculations and experimental validation. Our theoretical analysis of doped RuO2 catalysts reveals that heteroatom doping (Ni, Cu, and Zn) induces significant local charge transfer, leading to the increased charge state of Ru and the downshifted d-band center. This, in turn, enables the mechanism switching from the conventional AEM to the more efficient LOM, and finally improves OER activity. We also establish a simple yet powerful descriptor, Ne of Ru (representing charge density of Ru sites), which enables accurate prediction of both catalytic activity and stability. Guided by these theoretical predictions, we successfully synthesize a Ni-doped RuO2 catalyst, which exhibits excellent OER activity and stability in acidic media, achieving an overpotential of just 156 mV and maintaining stability for 4000 h at 10 mA cm−2, significantly surpassing the performance of the commercial RuO2. These findings not only provide fundamental insights into the mechanism-switching behavior in OER catalysis but also offer a practical strategy for designing high-performance, stable electrocatalysts for acidic water electrolysis.
基于ruo2的高效PEM电解催化剂中局部电荷再分配诱导的OER机制切换
析氧反应(OER)是公认的水电解的瓶颈。为了确定潜在的反应机制,特别是吸附质演化机制(AEM)和晶格-氧参与机制(LOM)的相对贡献,我们结合密度泛函理论(DFT)计算和实验验证进行了全面的研究。我们对掺杂的RuO2催化剂的理论分析表明,杂原子掺杂(Ni, Cu和Zn)引起了显著的局部电荷转移,导致Ru的电荷态增加,d带中心下降。这反过来又使机制从传统的AEM切换到更高效的LOM,并最终改善OER活动。我们还建立了一个简单而强大的描述符,Ne of Ru(代表Ru位点的电荷密度),它可以准确预测催化活性和稳定性。在这些理论预测的指导下,我们成功地合成了一种ni掺杂的RuO2催化剂,该催化剂在酸性介质中表现出优异的OER活性和稳定性,在10 mA cm−2下实现了156 mV的过电位,并保持了4000 h的稳定性,大大超过了商用RuO2的性能。这些发现不仅为OER催化的机制转换行为提供了基本的见解,而且为设计高性能、稳定的酸性水电解电催化剂提供了实用的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


