Rationally designed nickel-cobalt oxide/sulfide heterostructure for high-performance oxygen evolution reaction and anion exchange membrane water electrolysis
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
To realize the practical application of anion exchange membrane water electrolysis (AEMWE), it is essential to develop highly active, durable, and cost-effective electrocatalyst for oxygen evolution reaction (OER). Herein, we report a hollow-structured NixCo1−xO/Ni3S2/Co9S8 heterostructure synthesized via sequential template-assisted growth, thermal oxidation, and controlled sulfidation process. The abundant bimetallic heterointerfaces not only provide additional active sites but also promote electronic modulation via charge redistribution. Additionally, the porous and hollow architecture enhances active surface area and mass transfer ability, thereby increasing the number of accessible active sites for alkaline OER. As a result, the prepared electrocatalyst achieves low overpotential of 310 mV at 10 mA cm−2 and small Tafel slope of 55.94 mV dec−1, demonstrating the exceptional electrocatalytic performance for alkaline OER. When integrated as the anode in an AEMWE cell, it delivers outstanding performance with only 1.657 V at 1.0 A cm−2 and reaches high current density of 5.0 A cm−2 at 1.989 V, surpassing those of commercial RuO2. The cell also shows excellent long-term durability over 100 h with minimal degradation. This study highlights the strong potential of rationally engineered oxide/sulfide heterostructures for next-generation alkaline water electrolysis.
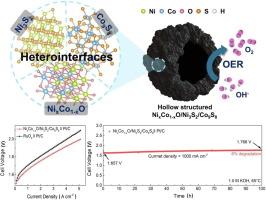
合理设计镍钴氧化物/硫化物异质结构,用于高性能析氧反应和阴离子交换膜电解
为了实现阴离子交换膜电解(AEMWE)的实际应用,必须开发出高效、耐用、经济的析氧反应(OER)电催化剂。本文报道了一种空心结构NixCo1−xO/Ni3S2/Co9S8异质结构,通过顺序模板辅助生长、热氧化和控制硫化工艺合成。丰富的双金属异质界面不仅提供了额外的活性位点,而且通过电荷再分配促进了电子调制。此外,多孔和中空的结构增强了活性表面积和传质能力,从而增加了碱性OER可达活性位点的数量。结果表明,所制备的电催化剂在10 mA cm−2下的过电位为310 mV, Tafel斜率为55.94 mV dec−1,具有良好的碱性OER电催化性能。在AEMWE电池中作为阳极集成时,它在1.0 A cm−2时的电流密度仅为1.657 V,在1.989 V时达到5.0 A cm−2的高电流密度,超过了商用RuO2。电池还表现出优异的长期耐久性超过100小时,降解最小。这项研究强调了合理设计的氧化物/硫化物异质结构在下一代碱性电解中的强大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


