Inhibitory modulation of gastrointestinal motility in mice by Tyr-c[D-Lys-Gly-p-F-Phe-Asp]-D-Pro-NH2, a novel cyclic hexapeptide with multifunctional opioid agonism
IF 2.7
3区 医学
Q3 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Opioid analgesics are critical for managing moderate-to-severe pain,yet are limited by adverse gastrointestinal (GI) effects, notably constipation. This necessitates developing novel opioid agonists with robust analgesia and reduced GI side effects. The cyclic hexapeptide Tyr-c[D-Lys-Gly-p-F-Phe-Asp]-D-Pro-NH2 (analog 15), a recently characterized multifunctional agonist of μ-opioid receptor (MOR), κ-opioid receptor (KOR), and δ-opioid receptor (DOR), exhibits potent antinociception following subcutaneous (s.c.) administration with constipation observed only at high doses. To further evaluate its GI impact, we assessed the effects of analog 15 on intestinal motility using in vivo upper GI transit and colonic bead expulsion assays. Our results indicated that fentanyl, analog 15, and its parent peptide analog 0 dose-dependently slowed upper GI transit and colonic expulsion after s.c. administration, with the upper GI tract exhibiting greater sensitivity. Mechanistically, fentanyl inhibited the GI motility via both central and peripheral opioid receptors, whereas analog 15 inhibited upper GI transit exclusively through the peripheral MOR, KOR, and DOR, and suppressed colonic transit via the peripheral MOR and KOR, both effects were independent of the central opioid receptor pathway. In conclusion, we demonstrated that the high doses of analog 15 inhibited GI motility through peripherally restricted activation of multiple opioid receptors. This finding aligns with analog 15's limited blood-brain barrier (BBB) permeability, which explains its reduced constipating effects while preserving potent analgesia, thereby supporting the therapeutic potential of multi-target peripheral opioid agonists.
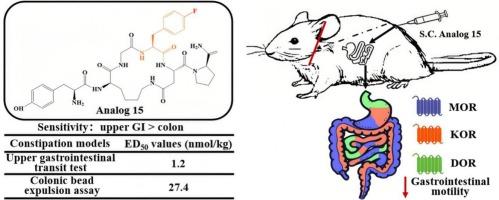
具有多功能阿片激动作用的新型环六肽Tyr-c[d - lys - gly -p- f - ph - asp]-D-Pro-NH2对小鼠胃肠运动的抑制调节
阿片类镇痛药对于治疗中度至重度疼痛至关重要,但其不良胃肠道(GI)效应(尤其是便秘)有限。这就需要开发具有强大镇痛作用和减少胃肠道副作用的新型阿片类激动剂。环六肽Tyr-c[D-Lys-Gly-p-F-Phe-Asp]-D-Pro-NH2(类似物15)是最近发现的μ-阿片样受体(MOR)、κ-阿片样受体(KOR)和δ-阿片样受体(DOR)的多功能激动剂,在皮下(s.c)给药后显示出有效的抗炎作用,仅在高剂量下观察到便秘。为了进一步评估其对胃肠道的影响,我们通过体内上消化道运输和结肠珠排出试验评估了类似物15对肠道运动的影响。我们的研究结果表明,芬太尼、类似物15及其母体肽类似物0剂量依赖性地减缓了s.c.给药后的上消化道转运和结肠排出,且上消化道表现出更大的敏感性。在机制上,芬太尼通过中枢和外周阿片受体抑制胃肠道运动,而类似物15仅通过外周MOR、KOR和DOR抑制上消化道运输,并通过外周MOR和KOR抑制结肠运输,这两种作用都独立于中枢阿片受体途径。总之,我们证明了高剂量的类似物15通过外周限制多种阿片受体的激活来抑制胃肠道运动。这一发现与类似物15有限的血脑屏障(BBB)渗透性一致,这解释了它在保持有效镇痛的同时减少便秘作用,从而支持多靶点外周阿片受体激动剂的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropeptides
医学-内分泌学与代谢
CiteScore
5.40
自引率
6.90%
发文量
55
审稿时长
>12 weeks
期刊介绍:
The aim of Neuropeptides is the rapid publication of original research and review articles, dealing with the structure, distribution, actions and functions of peptides in the central and peripheral nervous systems. The explosion of research activity in this field has led to the identification of numerous naturally occurring endogenous peptides which act as neurotransmitters, neuromodulators, or trophic factors, to mediate nervous system functions. Increasing numbers of non-peptide ligands of neuropeptide receptors have been developed, which act as agonists or antagonists in peptidergic systems.
The journal provides a unique opportunity of integrating the many disciplines involved in all neuropeptide research. The journal publishes articles on all aspects of the neuropeptide field, with particular emphasis on gene regulation of peptide expression, peptide receptor subtypes, transgenic and knockout mice with mutations in genes for neuropeptides and peptide receptors, neuroanatomy, physiology, behaviour, neurotrophic factors, preclinical drug evaluation, clinical studies, and clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


