Urea-functionalized ionic polymeric materials with adjustable ion density for efficient CO2 fixation and iodine adsorption
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
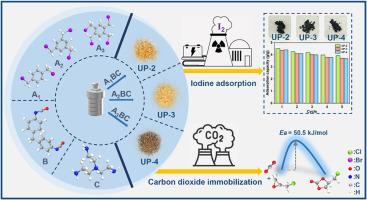
Ionic polymeric materials have unique physical and chemical properties that make them crucial in the fields of catalysis and adsorption. This study successfully synthesized a series of urea-functionalized ionic polymeric materials, designated as UP-x (x = 2, 3, 4). The synthesis employed a simple solvothermal method in conjunction with a three-component condensation and the Menshutkin reaction, facilitating the precise incorporation of highly active urea units and the intentional modulation of ion density by varying the connecting monomers. The functional groups, microstructure, thermal stability and pore structure of the materials were characterized using FT-IR, 13C NMR, SEM, TGA and BET. Subsequently, these materials were applied in CO2 fixation and iodine adsorption. All synthesized materials demonstrated effective catalytic activity, with UP-4 achieving a yield of up to 91.3 %. Experimental investigations have demonstrated that UP-4 is recyclable and can accommodate a wide range of substrates. Kinetic analyses revealed a reduction in the activation energy of the reaction to 50.5 kJ/mol. UP-x (x = 2, 3, 4) exhibited exceptional performance in the adsorption of iodine vapors, achieving maximum adsorption capacities of 4.51 g/g, 4.30 g/g, and 4.40 g/g, respectively. Results from DFT calculation confirm that the urea groups in the polymer framework can effectively activate epoxides and CO2 molecules, thereby enhancing the cycloaddition reaction. The urea units, bromine ions, and aromatic rings serve as iodine adsorption sites, enabling efficient iodine capture. The adsorption mechanism of iodine vapor on these materials involves both physisorption and chemisorption.
具有可调离子密度的尿素功能化离子聚合物材料,用于高效的CO2固定和碘吸附
离子高分子材料具有独特的物理和化学性质,在催化和吸附领域具有重要意义。本研究成功合成了一系列脲功能化离子高分子材料,命名为UP-x (x = 2,3,4)。该合成方法采用简单的溶剂热法,结合三组分缩合和Menshutkin反应,促进了高活性尿素单元的精确结合,并通过改变连接单体来有意调节离子密度。采用FT-IR、13C NMR、SEM、TGA和BET对材料的官能团、微观结构、热稳定性和孔隙结构进行了表征。随后,将这些材料应用于CO2固定和碘吸附。所有合成材料均表现出有效的催化活性,其中up -4的产率高达91.3%。实验研究表明UP-4是可回收的,可以容纳广泛的衬底。动力学分析表明,反应的活化能降低到50.5 kJ/mol。UP-x (x = 2,3,4)对碘蒸气的吸附性能优异,最大吸附量分别为4.51 g/g、4.30 g/g和4.40 g/g。DFT计算结果证实,聚合物框架中的尿素基团能有效激活环氧化物和CO2分子,从而增强环加成反应。尿素单元,溴离子和芳香环作为碘吸附位点,使碘有效捕获。碘蒸气在这些材料上的吸附机理包括物理吸附和化学吸附。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


