Clay catalyzed dihydropyrimidinone synthesis: α-glucosidase inhibition and chemoinformatics
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
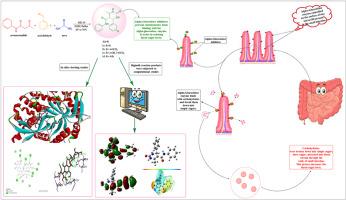
This research focuses on the synthesis of 1,2,3,4-tetrahydropyrimidine-5-carboxamide derivatives via the Biginelli reaction, a renowned one-pot MCR that employs three key reactants a β-keto ester, an aryl aldehyde and urea. In the current study, employing Montmorillonite K-10, a heterogeneous green clay catalyst led to a faster reaction and a yield of 85–93 %. This catalyst offers a large surface area, enhancing reactants efficiency and accelerating the reaction rate. Molecular structures of the target compounds were elucidated using FT-IR, 1H NMR, 13C NMR and Mass spectrometry. The HOMO-LUMO energy gap, MEP values and vibrational frequencies were investigated through DFT studies to evaluate reactivity and for theoretical-experimental correlations. Using ADMETlab 2.0, ADMET properties were examined to determine the drug-likeness of the targets. In-silico docking simulations were performed to analyze ligand-enzyme interactions and assess the binding affinity of target compounds toward the α-Glucosidase receptor. Synthesized compounds were screened for anti-diabetic activity using in-vitro α-Glucosidase Inhibition Assay.
粘土催化合成二氢嘧啶酮:α-葡萄糖苷酶抑制和化学信息学
本研究主要通过Biginelli反应合成1,2,3,4-四氢嘧啶-5-羧基酰胺衍生物。Biginelli是一种著名的单锅MCR反应,采用三种关键反应物:β-酮酯、芳基醛和尿素。在本研究中,采用异相绿粘土催化剂蒙脱土K-10,反应速度更快,收率为85 - 93%。这种催化剂提供了一个大的表面积,提高了反应物的效率,加快了反应速度。利用FT-IR、1H NMR、13C NMR和质谱对目标化合物的分子结构进行了分析。通过DFT研究了HOMO-LUMO能隙、MEP值和振动频率,以评估反应性和理论-实验相关性。使用ADMETlab 2.0,检测ADMET的性质以确定靶标的药物相似性。通过计算机对接模拟分析配体与酶的相互作用,并评估目标化合物与α-葡萄糖苷酶受体的结合亲和力。采用体外α-葡萄糖苷酶抑制试验筛选合成化合物的抗糖尿病活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


