Mineral carbonation of flue gas desulfurization gypsum to produce solid nano carbonates for building materials and allied characterizations
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
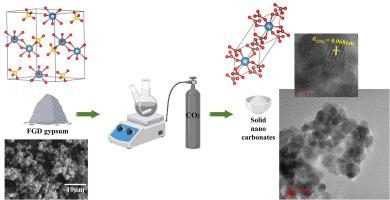
This study evaluates the mineral carbonation of flue gas desulfurization (FGD) gypsum to produce solid nano carbonates for building materials and allied characterizations. Solid nano calcium carbonate is produced within 60 min with the application of NaOH and acetone for improving reaction stability and achieving high concentration calcite (CaCO3) particles. This study details the optimization of a method for synthesizing nano CaCO3 from FGD gypsum and CO2 at ambient temperature and atmospheric pressure. In contrast to previous research, this methodology incorporates acetone as a stabilizing agent to inhibit re-dissolution and facilitate the creation of high purity nano calcite. The impact of the selected process on the characteristics of CaCO3 particles is assessed using Thermal gravimetric analysis and differential thermal analysis (TGA-DTA), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy-energy dispersive spectroscopy (FESEM-EDS) and High resolution transmission electron microscopy (HRTEM) techniques. 1 M NaOH solution sample (C1) has shown the optimum conversion of FGD gypsum into solid nano carbonates with 84.08 % CaCO3 purity, 87.67 % carbonation rate and 94.01 % carbonation efficiency. The mean particle size of produced nano carbonate is found to be 45.72 nm with rhomboidal and spherical shapes. The selected area electron diffraction (SAED) of solid nano carbonates sample showed a distinct spot pattern, indicating its single crystalline nature. This work proposed an advantageous solution, as it facilitates both CO2 fixation and the generation of solid nano calcium carbonate particles for application in building materials. Future research should focus on the mineral carbonation utilizing various feedstocks, the advancement of efficient processes and the implementation of mineral carbonation at the pilot scale from the perspectives of waste management and circular economy.
烟气脱硫石膏矿物碳化生产建筑材料用固体纳米碳酸盐及相关表征
本研究评估了矿物碳化的烟气脱硫(FGD)石膏生产固体纳米碳酸盐的建筑材料和相关的表征。为了提高反应稳定性,获得高浓度的碳酸钙(CaCO3)颗粒,在NaOH和丙酮的作用下,在60 min内制备出固体纳米碳酸钙。研究了在常温常压下以脱硫石膏和CO2为原料合成纳米碳酸钙的工艺条件。与之前的研究相比,该方法采用丙酮作为稳定剂来抑制再溶解,促进高纯度纳米方解石的产生。采用热重分析和差热分析(TGA-DTA)、x射线衍射(XRD)、傅里叶变换红外光谱(FTIR)、场发射扫描电子显微镜-能谱(FESEM-EDS)和高分辨率透射电子显微镜(HRTEM)技术评估了所选工艺对CaCO3颗粒特性的影响。在1 M NaOH溶液样品(C1)中,脱硫石膏转化为固体纳米碳酸盐的效果最佳,CaCO3纯度为84.08%,碳化率为87.67%,碳化效率为94.01%。所得纳米碳酸盐的平均粒径为45.72 nm,呈菱形和球形。固体纳米碳酸盐样品的选择区电子衍射(SAED)显示出明显的斑点图案,表明其单晶性质。这项工作提出了一个有利的解决方案,因为它既有利于二氧化碳的固定,又有利于固体纳米碳酸钙颗粒的产生,可用于建筑材料。未来的研究应从废物管理和循环经济的角度出发,着重研究利用多种原料的矿物碳酸化、推进高效的工艺和在中试规模上实施矿物碳酸化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


