Study on the synergistic corrosion inhibition effect of metal ion modified citrus peel extract on Q235 carbon steel in hydrochloric acid
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Corrosion inhibitors extracted from plants have good prospects for development, but the lack of outstanding corrosion inhibition efficiency is a serious problem. Therefore, in this paper, the extract of orange peel was used as a green corrosion inhibitor (Org), and in order to obtain excellent corrosion inhibition effect, it was combined with FeCl3 and TiCl4 to form two nanocomposite inhibitors, Org/Fe and Org/Ti, respectively. The corrosion inhibition performance and mechanism of the three corrosion inhibitors were investigated on carbon steel in 1.0 mol/L HCl solution. The weight loss and electrochemical results showed that the corrosion inhibition efficiencies of Org/Fe and Org/Ti were higher than that of Org, which indicated that Ti4+ and Fe3+ could effectively improve the corrosion inhibition performance of Org. The adsorption of Org, Org/Fe and Org/Ti on Q235 indicated that the adsorption belonged to Langmuir isothermal adsorption. The results of particle size analysis showed that Org/Fe and Org/Ti belonged to nanocomposite inhibitors. Theoretical calculations indicate that the addition of metal ions forms a dense protective film, thereby more effectively isolating the corrosive medium.
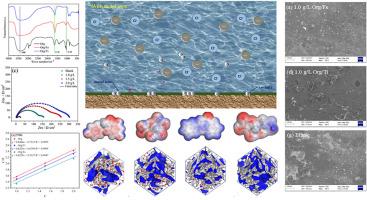
金属离子改性柑橘皮提取物对Q235碳钢在盐酸中的协同缓蚀作用研究
从植物中提取的缓蚀剂具有良好的开发前景,但缓蚀效果不突出是一个严重的问题。因此,本文将橙皮提取物作为绿色缓蚀剂(Org),为了获得优异的缓蚀效果,将其与FeCl3和TiCl4结合,分别形成两种纳米复合缓蚀剂Org/Fe和org/tii。研究了3种缓蚀剂在1.0 mol/L HCl溶液中对碳钢的缓蚀性能及机理。失重和电化学结果表明,Org/Fe和Org/Ti的缓蚀效率高于Org,表明Ti4+和Fe3+可以有效提高Org的缓蚀性能。Org、Org/Fe和Org/Ti在Q235上的吸附表明,吸附属于Langmuir等温吸附。粒度分析结果表明,Org/Fe和Org/Ti属于纳米复合抑制剂。理论计算表明,金属离子的加入可形成致密的保护膜,从而更有效地隔离腐蚀介质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


