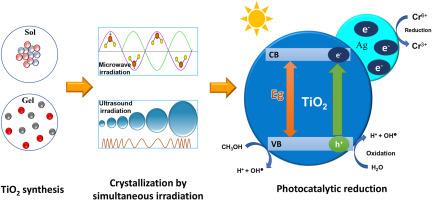
Simultaneous microwave-ultrasound irradiation of Ag/TiO2, synthesis for photoreduction of Cr(VI) using methanol as sacrificial agent
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this work, TiO2 and Ag/TiO2 composites powders were synthesized via the sol-gel method assisted by a novel simultaneous microwave-ultrasound irradiation during the crystallization step. The Ag content in the samples, determined by energy-dispersive spectroscopy coupled with scanning electron microscopy (SEM-EDS), was consistent with the theoretical loadings, yielding values of 1.0 % and 4.5 % for the composites. Surface atomic percentages obtained by X-ray photoelectron spectroscopy (XPS) were 0.3 % and 0.5 %. Raman spectroscopy revealed the characteristic peaks of anatase and brookite phases, while by X-ray diffraction confirmed the crystalline phases of TiO2 and detected signals associated with Ag. The specific surface areas of the photocatalysts ranged from 89 to 126 m2/g. The band gap energies determined from UV–Vis using the Kubelka-Munk function, were 3.0 eV for both composites. Photocatalytic performance was assessed through the photoreduction of Cr(VI) using methanol as a sacrificial agent under UV light (365 nm). Complete photoreduction of Cr(VI) to Cr(III) was achieved within 120 min using the 5 % Ag sample. In this system, Ag acted as electron traps, enhancing charge separation and thereby improving photocatalytic activity.
微波超声同时照射Ag/TiO2,以甲醇为牺牲剂合成光还原Cr(VI)
本文采用溶胶-凝胶法合成了TiO2和Ag/TiO2复合粉体,并在结晶过程中采用了一种新型的微波-超声同步照射。通过能量色散光谱和扫描电子显微镜(SEM-EDS)测定样品中的Ag含量,其屈服值分别为1.0%和4.5%,与理论载荷一致。x射线光电子能谱(XPS)测得的表面原子率分别为0.3%和0.5%。拉曼光谱显示出锐钛矿和板岩相的特征峰,x射线衍射证实了TiO2的晶相,并检测到与Ag相关的信号。光催化剂的比表面积为89 ~ 126 m2/g。利用Kubelka-Munk函数从UV-Vis测定的带隙能均为3.0 eV。以甲醇为牺牲剂,在365 nm紫外光下光还原Cr(VI),评价其光催化性能。使用5% Ag样品,在120分钟内实现了Cr(VI)到Cr(III)的完全光还原。在该体系中,银作为电子陷阱,促进电荷分离,从而提高光催化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


