Efficient cadmium removal from phosphoric acid by adsorption onto functionalized bentonite and activated carbon derived from rosemary root
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cadmium content in wet phosphoric acid remains a major challenge in the industrial production of phosphate fertilizers. This study investigates the removal of cadmium using two novel adsorbent materials: modified bentonite clay and activated carbon derived from rosemary root. The bentonite clay was functionalized by intercalating silver hydroxide (Ag(OH)) between its layers, followed by grafting diethyl O,O-dithiophosphoric ester (-SH group) onto its surface. Activated carbon was prepared via chemical carbonization of rosemary root using phosphoric acid. The adsorbents were characterized using XRD, SEM, EDX/EDS, and BET techniques, and their cadmium adsorption performance was evaluated in a batch system. A full factorial design was employed to study the effects of adsorbent specific mass, phosphoric acid concentration (P2O5 %), and temperature on cadmium removal efficiency. The results showed that modified bentonite achieved up to 89 % cadmium removal at a specific mass of 6 g per 100 g of wet phosphoric acid, particularly at P2O5 concentrations between 6.8 % and 12.6 %, with higher pH favoring improved performance. Activated carbon achieved a maximum removal efficiency of approximately 70 % at a specific mass of 1 g per 100 g of wet phosphoric acid for P2O5 concentrations below 10 %. The enhanced performance of modified bentonite is attributed to chemisorption through covalent bonding between cadmium ions and surface sulfur groups. These findings highlight the potential of functionalized bentonite as an effective and economical adsorbent for cadmium removal in industrial phosphoric acid processing.
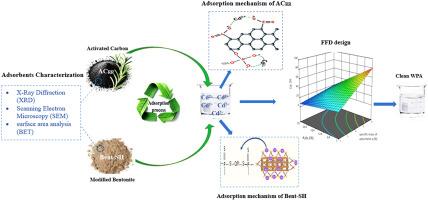
迷迭香根基功能化膨润土和活性炭对磷酸中镉的高效吸附
湿磷酸中镉的含量一直是磷肥工业生产中面临的主要挑战。研究了改性膨润土和迷迭香根活性炭两种新型吸附材料对镉的去除效果。将氢氧化银(Ag(OH))嵌入膨润土层间,接枝O,O-二硫代磷酸二乙酯(-SH基团)在膨润土层间实现功能化。以迷迭香根为原料,采用磷酸化学炭化法制备活性炭。采用XRD、SEM、EDX/EDS和BET技术对吸附剂进行了表征,并在批处理体系中对其镉吸附性能进行了评价。采用全因子设计研究了吸附剂比质量、磷酸浓度(P2O5 %)和温度对镉去除率的影响。结果表明,改性膨润土在湿磷酸比质量为6 g / 100 g时,除镉率高达89%,特别是在P2O5浓度为6.8% ~ 12.6%时,pH值越高,除镉率越高。当P2O5浓度低于10%时,每100 g湿磷酸的比质量为1 g时,活性炭的最大去除效率约为70%。改性膨润土性能的增强是由于镉离子与表面硫基团之间的共价键作用引起的化学吸附。这些发现突出了功能化膨润土作为工业磷酸处理中有效和经济的除镉吸附剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


