Activity of two-dimensional Cr2CTx MXene for the synthesis of Eugenol-1,2,3-Triazole via click chemistry
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The development of efficient heterogeneous catalysts for the synthesis of heterocyclic compounds is crucial for various applications. In this work, two-dimensional Cr2CTx MXene was successfully synthesized via a selective etching method using HF and HCl. Its formation and structural properties were comprehensively verified using various characterization techniques, including XRD, FTIR, Raman spectroscopy, FE-SEM, TEM, and XPS. In addition, the as-prepared Cr2CTx MXene was used as a catalyst support for the synthesis of eugenol-1,2,3-triazole via azide-alkyne cycloaddition reaction. Optimal reaction conditions, utilizing 10 wt% Cr2CTx MXene at 80 °C for 17 h, resulted in a 33 % yield, where both intermediate compounds and the final product were comprehensively characterized by FTIR, 1H NMR, 13C NMR, and LC-MS/MS. Besides, Cr2CTx MXene catalyst was successfully reused for up to three cycles, demonstrating excellent durability and maintaining high product yields without significant loss of activity. Furthermore, the synthesized eugenol-1,2,3-triazole exhibited significant antioxidant activity in the DPPH assay, with 80 % radical scavenging at a concentration of 1000 μg/mL. This finding offers novel insights into the catalytic potential of Cr2CTx MXene for efficient organic transformations, yielding compounds with promising antioxidant properties.
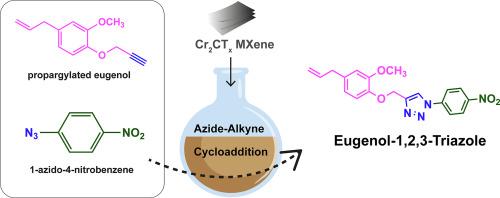
二维Cr2CTx MXene在点击化学合成丁香酚1,2,3-三唑中的活性
开发高效的多相催化剂来合成杂环化合物对于各种应用都是至关重要的。本文采用HF和HCl选择性蚀刻法制备了二维Cr2CTx MXene。采用XRD、FTIR、拉曼光谱、FE-SEM、TEM、XPS等多种表征技术对其形成和结构性能进行了全面验证。此外,制备的Cr2CTx MXene作为催化剂载体,通过叠氮-炔环加成反应合成了丁香酚-1,2,3-三唑。最佳反应条件是在80°C条件下,使用10 wt%的Cr2CTx MXene反应17 h,产率为33%,其中中间化合物和最终产物均通过FTIR, 1H NMR, 13C NMR和LC-MS/MS进行了全面表征。此外,Cr2CTx MXene催化剂成功地重复使用了多达三个循环,表现出优异的耐久性和保持高产品收率,而没有明显的活性损失。此外,合成的丁香酚-1,2,3-三唑在DPPH实验中表现出显著的抗氧化活性,浓度为1000 μg/mL时自由基清除率达80%。这一发现为Cr2CTx MXene在高效有机转化中的催化潜力提供了新的见解,从而产生了具有良好抗氧化性能的化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


